Co-targeting of the thymic stromal lymphopoietin receptor to decrease immunotherapeutic resistance in CRLF2-rearranged Ph-like and Down syndrome acute lymphoblastic leukemia
- PMID: 39681640
- PMCID: PMC11879877
- DOI: 10.1038/s41375-024-02493-3
Co-targeting of the thymic stromal lymphopoietin receptor to decrease immunotherapeutic resistance in CRLF2-rearranged Ph-like and Down syndrome acute lymphoblastic leukemia
Abstract
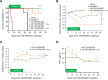
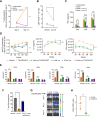
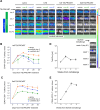
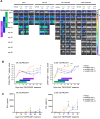
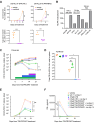
CRLF2 rearrangements occur in >50% of Ph-like and Down syndrome (DS)-associated B-acute lymphoblastic leukemia (ALL) and induce constitutive kinase signaling targetable by the JAK1/2 inhibitor ruxolitinib under current clinical investigation. While chimeric antigen receptor T cell (CART) immunotherapies have achieved remarkable remission rates in children with relapsed/refractory B-ALL, ~50% of CD19CART-treated patients relapse again, many with CD19 antigen loss. We previously reported preclinical activity of thymic stromal lymphopoietin receptor-targeted cellular immunotherapy (TSLPRCART) against CRLF2-overexpressing ALL as an alternative approach. In this study, we posited that combinatorial TSLPRCART and ruxolitinib would have superior activity and first validated potent TSLPRCART-induced inhibition of leukemia proliferation in vitro in CRLF2-rearranged ALL cell lines and in vivo in Ph-like and DS-ALL patient-derived xenograft (PDX) models. However, simultaneous TSLPRCART/ruxolitinib or CD19CART/ruxolitinib treatment during initial CART expansion diminished T cell proliferation, blunted cytokine production, and/or facilitated leukemia relapse, which was abrogated by time-sequenced/delayed ruxolitinib co-exposure. Importantly, ruxolitinib co-administration prevented fatal TSLPRCART cytokine-associated toxicity in ALL PDX mice. Upon ruxolitinib withdrawal, TSLPRCART functionality recovered in vivo with clearance of subsequent ALL rechallenge. These translational studies demonstrate an effective two-pronged therapeutic strategy that mitigates acute CART-induced hyperinflammation and provides potential anti-leukemia 'maintenance' relapse prevention for CRLF2-rearranged Ph-like and DS-ALL.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: AB is a current employee of Carisma Therapeutics. SLR is a current employee of Parexel International. KRR has received preclinical research support from Incyte Corporation. TJF was a prior part-time employee of and consultant for Sana Biotechnology. TJF is an inventor on patent US11834509B2 (‘Thymic stromal lymphopoietin receptor-specific chimeric antigen receptors and methods using same’). SKT receives clinical research funding from Incyte Corporation for conduction of the Children’s Oncology Group AALL1521 phase 2 clinical trial (NCT02723994). The remaining authors declare no competing interests. Ethics approval and consent to participate: Viably cryopreserved primary pediatric, adolescent, and young adult ALL specimens used to create PDX models for these studies were obtained from leukemia biorepositories of the Children’s Oncology Group, Children’s Hospital of Philadelphia (CHOP), or Texas Children’s Hospital under institutional review board (IRB)-approved research protocols following obtainment of informed consent in accordance with the Declaration of Helsinki. Use of coded leukemia specimens without identifying patient health information in these studies was deemed non-human subjects research and exempt from further review by the CHOP IRB and ethics committee. All animal studies were conducted under an Institutional Animal Care and Use Committee-approved protocol at CHOP in accordance with all guidelines and regulations.
Figures

References
-
- Tran TH, Tasian SK. How I Treat Philadelphia Chromosome-like Acute Lymphoblastic Leukemia in Children, Adolescents, and Young Adults. Blood. 2024 10.1182/blood.2023023153. - PubMed
-
- Tasian SK, Dai YF, Devidas M, Roberts KG, Harvey RC, Chen IML, et al. Outcomes of patients with CRLF2-overexpressing acute lymphoblastic leukemia without Down syndrome: a report from the Children’s Oncology Group. Blood. 2020;136:45–46.
MeSH terms
Substances
Grants and funding
- U01CA243072/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- W81XWH-19-1-0197/U.S. Department of Defense (United States Department of Defense)
- U01CA232486/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- U01 CA243072/CA/NCI NIH HHS/United States
- K12HD043245/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- W81XWH-19-1-0196/U.S. Department of Defense (United States Department of Defense)
- T32HD043021/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- K12 HD043245/HD/NICHD NIH HHS/United States
- T32 CA009615/CA/NCI NIH HHS/United States
- T32 HD043021/HD/NICHD NIH HHS/United States
- U01 CA232486/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

