Treatment-related skin reactions in enfortumab vedotin as a surrogate marker of survival and treatment response
- PMID: 39681749
- PMCID: PMC11785594
- DOI: 10.1007/s10147-024-02672-3
Treatment-related skin reactions in enfortumab vedotin as a surrogate marker of survival and treatment response
Abstract
Background: Treatment-related skin reactions (TRSRs) induced by enfortumab vedotin (EV) targeting nectin-4 are among the most common adverse events. However, their association with survival and treatment response is poorly understood.
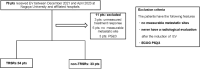
Methods: We retrospectively identified patients who received EV from December 2021 to April 2023 at Nagoya University Hospital and its affiliated facilities and extracted clinical data from their medical records. We evaluated cancer-specific survival (CSS) and progression-free survival (PFS) as survival outcomes and overall response rate (ORR) and disease control rate (DCR) as treatment responses between patients with and without TRSRs.
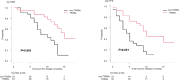
Results: In total, 67 eligible patients were identified. Thirty-four patients experienced TRSRs, and the remaining 33 did not experience TRSRs. The median follow-up period was 8 months. Patients in the TRSRs group demonstrated significantly longer median CSS (15 vs. 8 months; p = 0.003) and median PFS (10 vs. 5 months; p < 0.001) than the non-TRSRs. Regarding treatment response, the patients in the TRSRs group showed a favorable, albeit nonsignificant, treatment response trend compared with those in the non-TRSRs group (ORR, 73.5% vs. 51.5%; p = 0.107; DCR, 91.2 % vs. 81.8%; p = 0.444).
Conclusions: Patients with TRSRs demonstrated more prolonged survival and superior treatment responses to EV treatment. The role of TRSR as a surrogate marker of EV's efficacy should be further explored in prospective and sufficiently powered studies.
Keywords: Adverse effects; Bladder cancer; Enfortumab vedotin; Skin rash; Urothelial carcinoma.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: Shusuke Akamatsu received honoraria for lectures from Astellas. The remaining authors have declared no conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous