Baihui-Penetrating-Qubin Acupuncture Attenuates Neurological Deficits Through SIRT1/FOXO1 Reducing Oxidative Stress and Neuronal Apoptosis in Intracerebral Hemorrhage Rats
- PMID: 39682063
- PMCID: PMC11649584
- DOI: 10.1002/brb3.70095
Baihui-Penetrating-Qubin Acupuncture Attenuates Neurological Deficits Through SIRT1/FOXO1 Reducing Oxidative Stress and Neuronal Apoptosis in Intracerebral Hemorrhage Rats
Abstract
Background: Intracerebral hemorrhage (ICH) is a significant global disease with high mortality and disability. As of now, there is no effective therapy available. Oxidative stress and neuronal apoptosis play essential roles in ICH, determining neuronal survival. In our preliminary studies, we found that Baihui-penetrating-Qubin acupuncture could improve neurological deficits and neuropathological damage in the perihematomal area in ICH rats. The SIRT1/FOXO1 signaling pathway has been reported to mediate antioxidant and anti-neuronal apoptosis. This study aimed to investigate the effects of Baihui-penetrating-Qubin acupuncture on oxidative stress and neuronal apoptosis after ICH and the role of SIRT1/FOXO1 in acupuncture's neuroprotection.
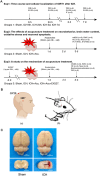
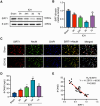
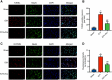
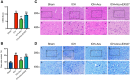
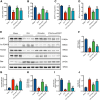
Methods: ICH rat models were established by autologous tail blood (50 µL) infusion into the caudate nucleus. EX527, SIRT1-specific inhibitor was intraperitoneally administered 3 days before ICH. Baihui-penetrating-Qubin acupuncture treatment was performed once a day for 30 min after ICH. Neurological deficits were evaluated using the modified neurological severity score (mNSS). Brain edema was evaluated using brain water content. HE staining and Nissl staining were used to evaluate neuropathological damage in the perihematomal area. Terminal deoxynucleotidyl transferase dUTP nick end labeling was used to quantify neuronal apoptosis. Specific kits were used to detect the levels of SOD, CAT, GSH-Px in the brain. The oxidative DNA damage was evaluated using enzyme-linked immunosorbent assay to detect the level of 8-hydroxyguanosine (8-OHdG). Western blot was used to evaluate the expressions of SIRT1, Ac-FOXO1, FOXO1, Bcl-2, and Bax. Immunofluorescence staining was conducted to detect the cellular localization of SIRT1.
Results: Baihui-penetrating-Qubin acupuncture improved the neurological deficits and brain edema, reduced the pathological injury and neuronal degeneration in 3 days in the perihematomal area after ICH. Mechanistically, acupuncture reduced oxidative stress injury and neuronal apoptosis via activating SIRT1/FOXO1 pathway. The neuroprotective effects of acupuncture were abolished by injection of the SIRT1 inhibitor EX527.
Conclusions: Baihui-penetrating-Qubin acupuncture could reduce oxidative stress and neuronal apoptosis, at least in part, through the SIRT1/FOXO1 signaling pathway, improving neurological deficits and neuropathological damage after ICH. These findings suggest that Baihui-penetrating-Qubin acupuncture is an effective therapy for ICH, as well as targeting SIRT1 signaling to promote neuron survival could be a potential therapeutic strategy.
Keywords: SIRT1/FOXO1; acupuncture; brain injury; intracerebral hemorrhage (ICH); neuronal apoptosis; neuroprotection; oxidative stress.
© 2024 The Author(s). Brain and Behavior published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Bederson, J. B. , Pitts L. H., Tsuji M., Nishimura M. C., Davis R. L., and Bartkowski H.. 1986. “Rat Middle Cerebral Artery Occlusion: Evaluation of the Model and Development of a Neurologic Examination.” Stroke; A Journal of Cerebral Circulation 17, no. 3: 472–476. 10.1161/01.str.17.3.472. - DOI - PubMed
-
- Chen, X. , Pan Z., Fang Z., et al. 2018. “Omega‐3 Polyunsaturated Fatty Acid Attenuates Traumatic Brain Injury‐induced Neuronal Apoptosis by Inducing Autophagy Through the Upregulation of SIRT1‐mediated Deacetylation of Beclin‐1.” Journal of Neuroinflammation 15, no. 1: 310. 10.1186/s12974-018-1345-8. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

