Salvage Therapy with Second-Generation Inhibitors for FLT3 Mutated Acute Myeloid Leukemia: A Real-World Study by the CETLAM and PETHEMA Groups
- PMID: 39682214
- PMCID: PMC11639769
- DOI: 10.3390/cancers16234028
Salvage Therapy with Second-Generation Inhibitors for FLT3 Mutated Acute Myeloid Leukemia: A Real-World Study by the CETLAM and PETHEMA Groups
Abstract
Background/objectives: Patients with relapsed/refractory (R/R) AML with FLT3 mutation (FLT3mut) have a dismal prognosis. FLT3mut offers a target for therapy in these patients. Gilteritinib (gilter) and quizartinib (quizar) have demonstrated efficacy as single agents in two phase 3 clinical trials.
Methods: We retrospectively analyzed the characteristics, treatments, and outcomes of 50 patients with R/R FLT3mut AML who received gilter or quizar as monotherapy in 27 Spanish centers before their commercial availability. Forty-four patients were treated with gilter and six with quizar.
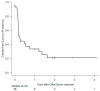
Results: The median age was 62.5 years, and 52% were women. Most patients presented with FLT3-ITD mutations (80%); 46% had refractory disease and 54% had relapsed disease at treatment initiation. First-line treatment was chemotherapy in 80% of patients, with 40% of these also receiving midostaurin. Twenty-five patients (50%) had previously received FLT3 inhibitor, and twenty-eight (56%) had received more than one line treatment before starting gilter/quizar. The rates of complete remission (CR), CR without hematological recovery (CRi), and partial remission were 22%, 18%, and 16%, respectively. The median overall survival (OS) and disease-free survival were 4.74 months and 2.99 months, respectively. We observed a significant improvement in OS in patients who had received only one prior line of therapy compared to those who had received two or more therapies (10.77 months vs. 4.24 months, p = 0.016). Multivariate analysis identified failure to achieve CR/CRi, receiving more than one prior line of therapy, age, and white blood cells count as independent prognostic factors for OS. The most common toxicities were febrile neutropenia, liver function abnormalities, and QT interval prolongation.
Conclusions: Gilter/quizar monotherapy are effective and tolerable options for patients with R/R FLT3mut AML in a real-world setting. Response and toxicity rates are similar to those reported in the phase 3 trials, despite the more heterogeneous nature of the study population.
Keywords: FLT3 mutations; acute myeloid leukemia; gilteritinib; quizartinib; real world data.
Conflict of interest statement
S.V.: Travel, accommodations, expenses from Astellas Pharma, Pfizer, Servier, AbbVie, and Jazz Pharmaceuticals; consulting or advisory role (without honoraria) for Astellas Pharma, Pfizer, Servier, AbbVie, and Jazz Pharmaceuticals. D.Q.: declares no conflicts of interest. M.M.: declares no conflicts of interest. I.CF.: declares no conflicts of interest. A.S.: declares no conflicts of interest. E.AC.: declares no conflicts of interest. M.C.: declares no conflicts of interest. M.DB.: declares no conflicts of interest. B.V.: declares no conflicts of interest. JA.RP.: Travel, accommodations, expenses from AbbVie, Beigene, Johnson & Johnson, Pfizer and Jazz Pharmaceuticals; consulting or advisory role (with honoraria) for Astellas Pharma. Speakers’ bureaus for AbbVie, Beigene and Astellas Pharma. M.A.: declares no conflicts of interest. A.G.: declares no conflicts of interest. A.B.: Travel, accommodations, expenses from Astellas Pharma, Pfizer, Servier, AbbVie, Gilead, and Jazz Pharmaceuticals; consulting or advisory role for Astellas Pharma, Pfizer, AbbVie, and Jazz Pharmaceuticals. AI.C.: declares no conflicts of interest. P.HP.: declares no conflicts of interest. J.S.: declares no conflicts of interest. R.C.: declares no conflicts of interest. M.T.: declares no conflicts of interest. J.LM.: declares no conflicts of interest. S.GA.: declares no conflicts of interest. MS.C.: declares no conflicts of interest. I.P.: declares no conflicts of interest. G.RM.: declares no conflicts of interest. M.C.: declares no conflicts of interest. A.P.: declares no conflicts of interest. A.H.: declares no conflicts of interest. M.T.: declares no conflicts of interest. L.C.: declares no conflicts of interest. MM.C.: declares no conflicts of interest. D.MC.: declares no conflicts of interest. J.E.: declares no conflicts of interest. P.M.: Consultancy or advisory role with Servier, Bristol Myers Squibb, and Novartis; speakers’ bureaus for Servier, Bristol Myers Squibb, Jazz Pharmaceuticals, Sanofi, AbbVie, and TEVA; research funding from Bristol Myers Squibb, AbbVie, and Daiichi Sankyo; travel, accommodations, or expenses from Pfizer.
Figures



References
-
- Arber D.A., Orazi A., Hasserjian R.P., Borowitz M.J., Calvo K.R., Kvasnicka H.M., Wang S.A., Bagg A., Barbui T., Branford S., et al. International consensus classifications of myeloid neoplasms and acute leukemias: Integrating morphologic, clinical, and genomic data. Blood. 2022;140:1200–1228. doi: 10.1182/blood.2022015850. - DOI - PMC - PubMed
-
- Khoury J.D., Solary E., Abla O., Akkari Y., Alaggio R., Apperley J.F., Bejar R., Berti E., Busque L., Chan J.K.C., et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia. 2022;36:1703–1719. doi: 10.1038/s41375-022-01613-1. - DOI - PMC - PubMed
-
- Döhner H., Wei A.H., Appelbaum F.R., Craddock C., DiNardo C.D., Dombret H., Ebert B.L., Fenaux P., Godley L.A., Hasserjian R.P., et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140:1345–1377. doi: 10.1182/blood.2022016867. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

