Carcinoembryonic Antigen Expression in Human Tumors: A Tissue Microarray Study on 13,725 Tumors
- PMID: 39682238
- PMCID: PMC11640007
- DOI: 10.3390/cancers16234052
Carcinoembryonic Antigen Expression in Human Tumors: A Tissue Microarray Study on 13,725 Tumors
Abstract
Background/objectives: Carcinoembryonic antigen (CEA) is a cell-surface glycoprotein serving as a drug target, diagnostic marker, and serum marker for cancer monitoring. However, prevalence data on CEA expression in cancer tissues vary considerably. This study was designed to determine CEA expression in normal and neoplastic tissues.
Methods: A tissue microarray containing 13,725 samples from 120 different tumor types, as well as 76 different normal tissue types, was analyzed by immunohistochemistry (IHC).
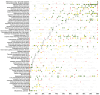
Results: CEA was detectable in 65 (54.2%) of 120 tumor categories, including 49 (40.8%) tumor types with at least one strongly positive case. CEA positivity was most common in colorectal adenomas (100%) and carcinomas (98.7%), other gastrointestinal adenocarcinomas (61.1-80.3%), medullary carcinomas of the thyroid (96.3%), pulmonary adenocarcinoma (73.7%), mucinous carcinomas of the ovary (79.8%) and the breast (43.2%), small-cell carcinomas of the lung (64.3%), and urinary bladder (38.9%). CEA overexpression was linked to high tumor grade and invasive growth (p < 0.0001 each) in urinary bladder cancer, and estrogen and HER2 receptor positivity (p ≤ 0.0158) in invasive breast cancer of no special type. In colorectal adenocarcinomas, reduced CEA expression was associated with mismatch repair deficiency (p < 0.0001).
Conclusions: The comprehensive list of CEA-positive human tumor types demonstrates that CEA is expressed in a broad range of epithelial neoplasms, many of which might benefit from CEA serum monitoring and anti-CEA therapies.
Keywords: CEA; diagnostic marker; human tumors; immunohistochemistry; tissue microarray.
Conflict of interest statement
The rabbit recombinant antibody, clone MSVA-465R was provided from MS Validated Antibodies GmbH (owned by a family member of GS). All other authors declare no conflicts of interest.
Figures




References
-
- Fakih M.G., Padmanabhan A. CEA monitoring in colorectal cancer. What you should know. Oncology. 2006;20:579–587; discussion 588, 594, 596. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

