Retrospective-Prospective Observational Study of Italian Patients Treated in Melanoma Adjuvant Cohort MAP-MADAM (Maximing ADjuvAnt MAP): Interim Analysis
- PMID: 39682258
- PMCID: PMC11640251
- DOI: 10.3390/cancers16234072
Retrospective-Prospective Observational Study of Italian Patients Treated in Melanoma Adjuvant Cohort MAP-MADAM (Maximing ADjuvAnt MAP): Interim Analysis
Abstract
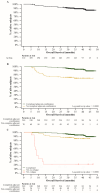
Background/Objective: Dabrafenib and trametinib (D + T) have been approved for the treatment of stage III melanoma with BRAF V600E V600K mutations in an adjuvant setting, based on the results from the COMBI-AD trial. To provide early access to this combination therapy prior to its commercial availability in Italy, a Managed Access Program (MAP) was run in Italy from June 2018 to December 2019. Methods: The MADAM (Maximing ADjuvAnt MAP) study is an Italian retrospective-prospective observational study that included patients who received at least one dose of D + T through the MAP. The primary endpoints were relapse-free survival (RFS) and overall survival (OS). Results: This interim analysis presents findings after the first 24 months of follow-up. A total of 310 patients were included in the study; 240 completed the 12-month treatment with D + T, while 70 discontinued the combination. RFS rates were 93.2% at 12 months and 80.2% at 24 months. The median RFS was not reached for the overall population or any subgroups. Similarly, the median OS was not reached, with OS rates of 96.4% at 12 months and 92.5% at 24 months. Conclusions: D + T achieved an RFS benefit, with effects sustained beyond the treatment period, indicating positive outcomes in this patient population.
Keywords: adjuvant; dabrafenib plus trametinib; melanoma; relapse-free survival; stage III.
Conflict of interest statement
I.M. and D.V. are employed by Novartis Farma S.p.A; M.D.V. has been advisor and/or consultant for B.M.S., M.S.D., Novartis, Pierre Fabre, Immunocore; F.G. reports personal fees for advisory role, speaker engagements and travel and accommodation expenses from Merck Sharp & Dohme, Novocure, Bristol Myers Squibb, Boehringer Ingelheim, Pharmamar, Novartis and Pierre Fabre; F.M. has been advisor for Novartis, B.M.S., Sunpharma, Sanofi, Pierre-Fabre, Ipsen and M.S.D.; F.S. declares to have received fees from B.M.S., M.S.D., Novartis, Pierre Fabre, Merck, Sanofi, Sun Pharma and has been advisor for M.S.D., Novartis, Pierre Fabre, Philogen; P.Q. received speaker fees and has been advisor for Novartis, B.M.S., M.S.D., Pierre Fabre; T.T. has been advisor for Amgen, Novartis, B.M.S., Roche, Servier, Pierre Fabre, M.S.D. No other COIs have been disclosed.
Figures



References
-
- Skin Cancer Facts & Statistics. 2022. [(accessed on 30 September 2024)]. Available online: https://www.skincancer.org/skin-cancer-information/skin-cancer-facts/
-
- GLOBOCAN Globocan. 2020. [(accessed on 30 September 2024)]. Available online: https://gco.iarc.fr/en.
-
- Gershenwald J.E., Soong S.J., Thompson J.F. Prognostic factors and natural history of melanoma. In: Balch C.M., Sober A.J., editors. Cutaneous Melanoma. 4th ed. Quality Medical Publishing; St. Louis, MO, USA: 2003.
-
- Garzon-Orjuela N., Prieto-Pinto L., Lasalvia P., Herrera D., Castrillon J., Gonzalez-Bravo D., Castaneda-Cardona C., Rosselli D. Efficacy and safety of dabrafenib-trametinib in the treatment of unresectable advanced/metastatic melanoma with BRAF-V600 mutation: A systematic review and network meta-analysis. Dermatol. Ther. 2020;33:e13145. doi: 10.1111/dth.13145. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

