Regorafenib Combined with BRAF/MEK Inhibitors for the Treatment of Refractory Melanoma Brain Metastases
- PMID: 39682270
- PMCID: PMC11640054
- DOI: 10.3390/cancers16234083
Regorafenib Combined with BRAF/MEK Inhibitors for the Treatment of Refractory Melanoma Brain Metastases
Abstract
Background: There are no active treatment options for patients with progressive melanoma brain metastases (MBM) failing immune checkpoint blockade (ICB) and BRAF/MEK inhibitors (BRAF/MEKi). Regorafenib (REGO), an oral multi-kinase inhibitor (incl. RAF-dimer inhibition), can overcome adaptive resistance to BRAF/MEKi in preclinical models.
Methods: This is a single-center retrospective case series of patients with refractory MBM treated with REGO plus BRAF/MEKi (compassionate use).
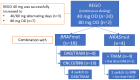
Results: A total of 22 patients were identified (18 BRAF-mutant, 4 NRASQ61-mutant; 19 with progressive MBM; 11 on corticosteroids). Thirteen BRAFV600-mutant patients were progressing on BRAF/MEKi at the time of REGO association. BRAF-mutant patients received REGO (40-80 mg once daily) combined with BRAF/MEKi, NRAS-mutant patients were treated with REGO + MEKi (+low-dose BRAFi to mitigate skin-toxicity). Grade 3 TRAE included arterial hypertension (n = 4) and maculopapular rash (n = 3). There were no G4/5 TRAE. In BRAF-mutant patients, overall and intracranial objective response rates (overall ORR and IC-ORR) were 11 and 29%, and overall and intracranial disease control rates (overall DCR and IC-DCR) were 44 and 59%, respectively. In NRAS-mutant patients overall ORR and IC-ORR were 0 and 25% and overall DCR and IC-DCR were 25 and 50%, respectively. The median PFS and OS were, respectively, 7.1 and 16.4 weeks in BRAF-mutant and 8.6 and 10.1 weeks in NRAS-mutant patients.
Conclusions: In heavily pretreated patients with refractory MBM, REGO combined with BRAF/MEKi demonstrated promising anti-tumor activity with an acceptable safety profile. In BRAFV600-mutant melanoma patients, responses cannot solely be attributed to BRAF/MEKi rechallenge. Further investigation in a prospective trial is ongoing to increase understanding of the efficacy.
Keywords: BRAF; BRAF/MEK inhibitors; NRAS; RAF dimer inhibitor; brain metastases; class II RAF inhibitor; regorafenib; stage IV-M1d melanoma; targeted therapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Cohen J.V., Tawbi H., Margolin K.A., Amravadi R., Bosenberg M., Brastianos P.K., Chiang V.L., de Groot J., Glitza I.C., Herlyn M. Melanoma central nervous system metastases: Current approaches, challenges, and opportunities. Pigment. Cell Melanoma Res. 2016;29:627–642. doi: 10.1111/pcmr.12538. - DOI - PMC - PubMed
-
- Dummer R., Flaherty K.T., Robert C., Arance A., de Groot J.W.B., Garbe C., Gogas H.J., Gutzmer R., Krajsová I., Liszkay G., et al. COLUMBUS 5-Year Update: A Randomized, Open-Label, Phase III Trial of Encorafenib Plus Binimetinib Versus Vemurafenib or Encorafenib in Patients With BRAF V600-Mutant Melanoma. J. Clin. Oncol. 2022;40:4178–4188. doi: 10.1200/JCO.21.02659. - DOI - PMC - PubMed
-
- Robert C., Grob J.J., Stroyakovskiy D., Karaszewska B., Hauschild A., Levchenko E., Chiarion Sileni V., Schachter J., Garbe C., Bondarenko I., et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N. Engl. J. Med. 2019;381:626–636. doi: 10.1056/NEJMoa1904059. - DOI - PubMed
-
- Wolchok J.D., Chiarion-Sileni V., Gonzalez R., Grob J.-J., Rutkowski P., Lao C.D., Cowey C.L., Schadendorf D., Wagstaff J., Dummer R., et al. CheckMate 067: 6.5-year outcomes in patients (pts) with advanced melanoma. J. Clin. Oncol. 2021;39:9506. doi: 10.1200/JCO.2021.39.15_suppl.9506. - DOI
-
- Long G.V., Atkinson V., Lo S., Guminski A.D., Sandhu S.K., Brown M.P., Gonzalez M., Scolyer R.A., Emmett L., McArthur G.A. Five-year overall survival from the anti-PD1 brain collaboration (ABC Study): Randomized phase 2 study of nivolumab (nivo) or nivo+ ipilimumab (ipi) in patients (pts) with melanoma brain metastases (mets) J. Clin. Oncol. 2021;39((Suppl. S15)):9508. doi: 10.1200/JCO.2021.39.15_suppl.9508. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

