Tolerability and Preliminary Outcomes of Adjuvant T-DM1 in HER2-Positive Breast Cancer After Neoadjuvant Therapy: The ATD Study
- PMID: 39682290
- PMCID: PMC11639934
- DOI: 10.3390/cancers16234104
Tolerability and Preliminary Outcomes of Adjuvant T-DM1 in HER2-Positive Breast Cancer After Neoadjuvant Therapy: The ATD Study
Abstract
Background/objectives: HER2-positive breast cancer (HER2+BC) is an aggressive subtype, with neoadjuvant treatment (NAT) aiming to achieve a pathological complete response (pCR) to improve long-term outcomes. Trastuzumab emtansine (T-DM1) has been established as the standard of care in the adjuvant setting for HER2+BC patients who do not obtain pCR. The ATD study aimed to evaluate the real-world tolerability of T-DM1 in this setting. The secondary objective was to assess the effectiveness.
Methods: This was a multicenter, retrospective study across 24 Italian oncology centers, including 410 patients with HER2+BC treated with adjuvant T-DM1 following a lack of pCR after NAT. Patient characteristics, NAT regimens, and surgical outcomes were recorded. Tolerability was assessed by documenting adverse events (AEs) according to the CTCAE (v5.0). Preliminary effectiveness was evaluated in terms of relapse-free survival (RFS) and overall survival (OS).
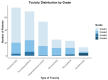
Results: Overall, 228 patients (55.6%) experienced at least one AE related to T-DM1, with 4.9% experiencing grade 3 or higher AEs. The most common AEs were hepatotoxicity (18.5%) and thrombocytopenia (17.6%). T-DM1 was discontinued in 10.0% of patients due to toxicity. After a median follow-up of 25 months, 31 relapse events (7.6%) and 22 deaths (5.4%) were reported. The preliminary incidence of RFS and OS events was similar between patients who completed the T-DM1 course and those who discontinued it early.
Conclusions: T-DM1 demonstrated a manageable safety profile, and the adverse events were consistent with those reported in randomized trials. The data are not yet sufficient to allow for a formal analysis of RFS and OS, and long-term follow-up is required.
Keywords: HER2-positive subtype; adjuvant T-DM1; adverse events; breast cancer; real-world evidence.
Conflict of interest statement
E.K. reports speaker fees/travel grants from Daiichi Sankyo, Novartis, and Takis Biotech, all outside the scope of this work. L.F. reports speaker fees/honoraria for writing engagement/travel grants from Daiichi Sankyo, Eisai, Gilead, and Istituto Gentili, all outside the scope of this work. F.S.D.L. reports speaker fees/travel grants from Daiichi Sankyo, Istituto Gentili, Novartis, Ipsen, and Pfizer, all outside the scope of this work. J.F. reports speaker fees/advisory boards from Menarini, AstraZeneca, and Novartis, all outside the scope of this work. I.M. reports occasional fees for advisory boards supported by Eli Lilly, Novartis, Pfizer, AstraZeneca, Daiichi Sankyo, Gilead, SeaGen, and Menarini StemLine, all outside the scope of this work. L.M. reports personal fees/advisory boards/travel grants from Pfizer, Eli Lilly, Daiichi Sankyo, Seagen, Roche, Novartis, Gilead, and Eisai, all outside the scope of this work. P.M. reports financial interests, personal and institutional, as a speaker, consultant, and advisor, from AstraZeneca, Boehringer Ingelheim, Incyte, and Novartis; financial interests, personal and as a speaker, consultant, and advisor, from Celgene, Eisai, and Merck; financial interests, personal, from a speaker’s bureau from Pierre Fabre; financial interests, personal and institutional, from advisory boards from Pfizer, Roche, Bristol Myers Squibb, and Gentech; financial interests, personal and for advisory boards, from Amgen and Merck Serono; and financial interests, institutional and research funding, from AstraZeneca, Bristol Myers Squibb, Incyte, Roche, Lilly, Merck, Novartis, and NanoString, all outside the scope of this work. A.B. reports personal fees for advisory/consultancy roles from Eli Lilly, Pfizer, Novartis, Roche, BMS, AstraZeneca, MSD, Daiichi Sankyo, Gilead, and Seagen, all outside the scope of this work. I.P. reports fees for advisory boards from Novartis, Roche, Eli Lilly, AstraZeneca, Daiichi Sankyo, Pfizer, Seagen, Italfarmaco, Istituto Gentili, and Genetic, all outside the scope of this work. M.M. (Marco Mazzotta) reports speaker fees/advisory boards/travel grants from AstraZeneca, Pfizer, Daiichi Sankyo, and Novartis, all outside the scope of this work. M.V. reports speaker fees/advisory boards/consultancy for AstraZeneca, Daiichi Sankyo, Istituto Gentili, and Novartis and travel grants from Novartis and Pfizer, all outside the scope of this work. G.D.: AstraZeneca, Daiichi Sankyo, Pfizer, Eli Lilly, Novartis, and Istituto Gentili, all outside the scope of this work. T.G.: Pfizer, Roche, AstraZeneca, Daiichi Sankyo, Novartis, and Istituto Gentili, all outside the scope of this work. F.G. reports travel grants/accommodation/congress registration from Pfizer, Daiichi Sankyo, Eli Lilly, and Novartis, all outside the scope of this work. F.P. (Francesco Pantano) reports advisory boards and speaker honoraria from AstraZeneca, Gilead, Novartis, Eli Lilly, Daiichi Sankyo, and Pfizer, all outside the scope of this work. G.T. reports advisory boards from Novartis Astra Zeneca e Molteni, all outside the scope of this work. A.F. (Agnese Fabbri): Eli Lilly, Novartis, Roche, Istituto Gentili, Pfizer, and AstraZeneca, all outside the scope of this work. E.B. has received grants or contracts from AstraZeneca, Roche, and honoraria for lectures from Merck-Sharp & Dome, AstraZeneca, Pfizer, Eli-Lilly, Bristol-Myers Squibb, Novartis, Takeda, and Roche and has been a member of the Data Safety Monitoring Board or Advisory Board of Merck-Sharp & Dome, Pfizer, Novartis, Bristol-Myers Squibb, AstraZeneca, Roche, and Celltrion, all outside the scope of this work. M.P. reports advisory board/consultant roles for Novartis, Eli Lilly, Pfizer, AstraZeneca, Daiichi Sankyo, and Accord and travel grants from Roche, all outside the scope of this work. R.B. reports advisory board and invited speaker roles for AstraZeneca, Bayer, Boeringher Ingelheim, Eisai, Gilead, Incyte, Eli Lilly, Menarini, Merck, and MSD and institutional funding from AstraZeneca, Pfizer, and Roche, all outside the scope of this work. A.I. reports consulting fees from Seagen, all outside the scope of this work. F.C. reports advisory board/consulting roles for Pfizer, BMS, Ipsen, MSD, AstraZeneca, Merck, Accord, Novartis, J&J, Astellas, and Eisai, all outside the scope of this work. L.P.: Novartis and Pfizer, all outside the scope of this work. P.V. reports speaker fees/advisory boards from Novartis, Ipsen, Daiichi Sankyo, MSD, Pfizer, and Eli Lilly; travel grants from Roche, Novartis, Ipsen, Pfizer, Daiichi and Sankyo; and institutional grants from Pfizer, Eisai, Novartis, and Daiichi Sankyo, all outside the scope of this work. The remaining authors have no conflicts of interest to declare.
Figures
References
-
- Slamon D.J., Leyland-Jones B., Shak S., Fuchs H., Paton V., Bajamonde A., Fleming T., Eiermann W., Wolter J., Pegram M., et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. - DOI - PubMed
-
- Cortazar P., Zhang L., Untch M., Mehta K., Costantino J.P., Wolmark N., Bonnefoi H., Cameron D., Gianni G., Valagussa P., et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. - DOI - PubMed
-
- von Minckwitz G., Untch M., Blohmer J.U., Costa S.D., Eidtmann H., Fasching P.A., Gerber B., Eiermann W., Hilfrich J., Huober J., et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous