Exploring the Immunoresponse in Bladder Cancer Immunotherapy
- PMID: 39682686
- PMCID: PMC11640729
- DOI: 10.3390/cells13231937
Exploring the Immunoresponse in Bladder Cancer Immunotherapy
Abstract
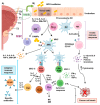
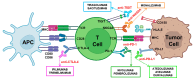
Bladder cancer (BC) represents a wide spectrum of diseases, ranging from recurrent non-invasive tumors to advanced stages that require intensive treatments. BC accounts for an estimated 500,000 new cases and 200,000 deaths worldwide every year. Understanding the biology of BC has changed how this disease is diagnosed and treated. Bladder cancer is highly immunogenic, involving innate and adaptive components of the immune system. Although little is still known of how immune cells respond to BC, immunotherapy with bacillus Calmette-Guérin (BCG) remains the gold standard in high-risk non-muscle invasive BC. For muscle-invasive BC and metastatic stages, immune checkpoint inhibitors targeting CTLA-4, PD-1, and PD-L1 have emerged as potent therapies, enhancing immune surveillance and tumor cell elimination. This review aims to unravel the immune responses involving innate and adaptive immune cells in BC that will contribute to establishing new and promising therapeutic options, while reviewing the immunotherapies currently in use in bladder cancer.
Keywords: BCG; bladder cancer; checkpoints; immune response; immunotherapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- The UroScreen Study Group. Pesch B., Taeger D., Johnen G., Gawrych K., Bonberg N., Schwentner C., Wellhäußer H., Kluckert M., Leng G., et al. Screening for bladder cancer with urinary tumor markers in chemical workers with exposure to aromatic amines. Int. Arch. Occup. Environ. Health. 2014;87:715–724. doi: 10.1007/s00420-013-0916-3. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- (PI1302297 and PI20_00161)/the Ministry of Economy and Competitiveness ISCIII-FIS
- (20812-PI-18)/Séneca Foundation, Science and Technology Agency from Murcia Region
- (AECC PRDMU21540RUIZ)./Robles Chillida Foundation (L.G.), University of Murcia, Campus Mare Nostrum and Spanish As-sociation Against Cancer
- I.R.-L. was funded by AECC.
LinkOut - more resources
Full Text Sources
Medical
Research Materials

