Targeted Deletion in the Basal Body Protein Talpid3 Leads to Loss of Primary Cilia in Embryonic Stem Cells and Defective Lineage-Specific Differentiation
- PMID: 39682705
- PMCID: PMC11639927
- DOI: 10.3390/cells13231957
Targeted Deletion in the Basal Body Protein Talpid3 Leads to Loss of Primary Cilia in Embryonic Stem Cells and Defective Lineage-Specific Differentiation
Abstract
Talpid3 is a basal body protein required for the formation of primary cilia, an organelle involved in signal transduction. Here, we asked if Talpid3 has a role in the regulation of differentiation and/or self-renewal of ES cells and whether cells lacking cilia due to a deletion in Talpid3 can be reprogrammed to induced pluripotent stem (iPS) cells. We show that mouse embryonic limb fibroblasts which lack primary cilia with a targeted deletion in the Talpid3 (Ta3) gene can be efficiently reprogrammed to iPS cells. Furthermore, vector-free Ta3-/- iPS cells retain ES cell features and are able to self-renew. However, both Ta3-/- iPS and ES cells are unable to form visceral endoderm and differentiate poorly into neurons. The observed defects are not a consequence of reprogramming since Ta3-/- ES cells also exhibit this phenotype. Thus, Talpid3 and primary cilia are required for some differentiation events but appear to be dispensable for stem cell self-renewal and reprogramming.
Keywords: differentiation; extraembryonic membranes; mouse ES cells; primary cilia; talpid 3.
Conflict of interest statement
The authors declare no competing interests.
Figures
 ), R1 (
), R1 ( ), Ta3−/− cells (
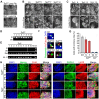
), Ta3−/− cells ( ). Error bars ± SEM, n ≥ 350 cells from three independent experiments. (H) Expression of pluripotency markers Nanog, Oct4, SSEA1 and Lin28 in Ta3fl/fl and Ta3−/− iPS cells. Scale bar: 25 µm.
). Error bars ± SEM, n ≥ 350 cells from three independent experiments. (H) Expression of pluripotency markers Nanog, Oct4, SSEA1 and Lin28 in Ta3fl/fl and Ta3−/− iPS cells. Scale bar: 25 µm.
 ), Ta3fl/fl iPS X12 and X22 (
), Ta3fl/fl iPS X12 and X22 ( ), Ta3fl/fl ES 9 (
), Ta3fl/fl ES 9 ( ), Ta3−/− iPS X10 and X17 (
), Ta3−/− iPS X10 and X17 ( ), and Ta3−/− ES 6 (
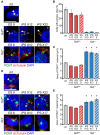
), and Ta3−/− ES 6 ( ). (E) Expression of the limb markers HoxD13 and Meis1 in ES and iPS cell lines and in E12.5 mouse limb (Lb,
). (E) Expression of the limb markers HoxD13 and Meis1 in ES and iPS cell lines and in E12.5 mouse limb (Lb,  ).
).
 ), Ta3−/− ES cells (
), Ta3−/− ES cells ( ), R1 (
), R1 ( ), Ta3fl/fl iPS (
), Ta3fl/fl iPS ( ), Ta3fl/fl ES cells (
), Ta3fl/fl ES cells ( ). (Error bars ±SEM, three independent experiments, ≥350 cells per experiment, * p < 0.05, one-way ANOVA with Tukey’s post-hoc). (D) Primary cilia in R1, transgene-free Ta3fl/fl X12 or X22 and Ta3−/− X10 and X17. ES cells immunostained for pericentriolar material 1 (Pcm1, hollow arrows) and acetylated tubulin (white arrows). Scale bar: 10 µm. (E) The mean area of pericentriolar material protein Pcm1 in immunostained images. Pcm1 areas in Ta3−/− iPS (
). (Error bars ±SEM, three independent experiments, ≥350 cells per experiment, * p < 0.05, one-way ANOVA with Tukey’s post-hoc). (D) Primary cilia in R1, transgene-free Ta3fl/fl X12 or X22 and Ta3−/− X10 and X17. ES cells immunostained for pericentriolar material 1 (Pcm1, hollow arrows) and acetylated tubulin (white arrows). Scale bar: 10 µm. (E) The mean area of pericentriolar material protein Pcm1 in immunostained images. Pcm1 areas in Ta3−/− iPS ( ), Ta3−/− ES cells (
), Ta3−/− ES cells ( ), R1 (
), R1 ( ), Ta3fl/fl iPS (
), Ta3fl/fl iPS ( ), Ta3fl/fl ES cells (
), Ta3fl/fl ES cells ( ). (Error bars ±SEM, three independent experiments, ≥350 cells per experiment, * p < 0.05, data were compared by one-way ANOVA with Tukey’s post-hoc). Scale bar: 10 µm.
). (Error bars ±SEM, three independent experiments, ≥350 cells per experiment, * p < 0.05, data were compared by one-way ANOVA with Tukey’s post-hoc). Scale bar: 10 µm.

 ), Ta3fl/fl ES 9(
), Ta3fl/fl ES 9( ), Ta3fl/fl iPS X12 (
), Ta3fl/fl iPS X12 ( ), Ta3fl/fl iPS X22 (
), Ta3fl/fl iPS X22 ( ), Ta3−/− ES 6 (
), Ta3−/− ES 6 ( ), Ta3−/− iPS X10 (
), Ta3−/− iPS X10 ( ) and Ta3−/− iPS 17 (
) and Ta3−/− iPS 17 ( ). n = 3. Error bars ± SEM. qPCRs were performed in triplicate for each time point. Data from 3 independent experiments.
). n = 3. Error bars ± SEM. qPCRs were performed in triplicate for each time point. Data from 3 independent experiments.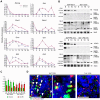

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

