Human Cytochrome P450 Cancer-Related Metabolic Activities and Gene Polymorphisms: A Review
- PMID: 39682707
- PMCID: PMC11639897
- DOI: 10.3390/cells13231958
Human Cytochrome P450 Cancer-Related Metabolic Activities and Gene Polymorphisms: A Review
Abstract
Background: Cytochromes P450 (CYPs) are heme-containing oxidoreductase enzymes with mono-oxygenase activity. Human CYPs catalyze the oxidation of a great variety of chemicals, including xenobiotics, steroid hormones, vitamins, bile acids, procarcinogens, and drugs.
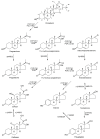
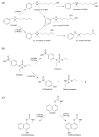
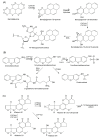
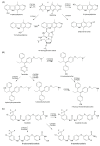
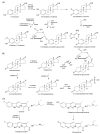
Findings: In our review article, we discuss recent data evidencing that the same CYP isoform can be involved in both bioactivation and detoxification reactions and convert the same substrate to different products. Conversely, different CYP isoforms can convert the same substrate, xenobiotic or procarcinogen, into either a more or less toxic product. These phenomena depend on the type of catalyzed reaction, substrate, tissue type, and biological species. Since the CYPs involved in bioactivation (CYP3A4, CYP1A1, CYP2D6, and CYP2C8) are primarily expressed in the liver, their metabolites can induce hepatotoxicity and hepatocarcinogenesis. Additionally, we discuss the role of drugs as CYP substrates, inducers, and inhibitors as well as the implication of nuclear receptors, efflux transporters, and drug-drug interactions in anticancer drug resistance. We highlight the molecular mechanisms underlying the development of hormone-sensitive cancers, including breast, ovarian, endometrial, and prostate cancers. Key players in these mechanisms are the 2,3- and 3,4-catechols of estrogens, which are formed by CYP1A1, CYP1A2, and CYP1B1. The catechols can also produce quinones, leading to the formation of toxic protein and DNA adducts that contribute to cancer progression. However, 2-hydroxy- and 4-hydroxy-estrogens and their O-methylated derivatives along with conjugated metabolites play cancer-protective roles. CYP17A1 and CYP11A1, which are involved in the biosynthesis of testosterone precursors, contribute to prostate cancer, whereas conversion of testosterone to 5α-dihydrotestosterone as well as sustained activation and mutation of the androgen receptor are implicated in metastatic castration-resistant prostate cancer (CRPC). CYP enzymatic activities are influenced by CYP gene polymorphisms, although a significant portion of them have no effects. However, CYP polymorphisms can determine poor, intermediate, rapid, and ultrarapid metabolizer genotypes, which can affect cancer and drug susceptibility. Despite limited statistically significant data, associations between CYP polymorphisms and cancer risk, tumor size, and metastatic status among various populations have been demonstrated.
Conclusions: The metabolic diversity and dual character of biological effects of CYPs underlie their implications in, preliminarily, hormone-sensitive cancers. Variations in CYP activities and CYP gene polymorphisms are implicated in the interindividual variability in cancer and drug susceptibility. The development of CYP inhibitors provides options for personalized anticancer therapy.
Keywords: CYPs; bioactivation vs. detoxification; drugs as substrates, inducers, and inhibitors; gene polymorphisms; hormone-dependent cancers; nuclear receptors and efflux transporters.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

