JRM-28, a Novel HDAC2 Inhibitor, Upregulates Plasticity-Associated Proteins in Hippocampal Neurons and Enhances Morphological Plasticity via Activation of CREB: Implications for Alzheimer's Disease
- PMID: 39682714
- PMCID: PMC11640089
- DOI: 10.3390/cells13231964
JRM-28, a Novel HDAC2 Inhibitor, Upregulates Plasticity-Associated Proteins in Hippocampal Neurons and Enhances Morphological Plasticity via Activation of CREB: Implications for Alzheimer's Disease
Abstract
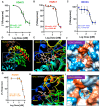
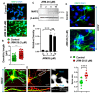
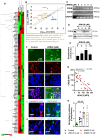
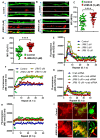
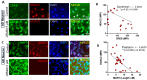
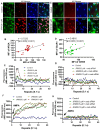
Enhancement of neuronal plasticity by small-molecule therapeutics protects cognitive skills and also ameliorates progressive neurodegenerative pathologies like Alzheimer's disease (AD) and dementia. One such compound, a novel histone deacetylase 2 (HDAC2) inhibitor named JRM-28, was shown here to enhance dendritic strength, augment spine density, and upregulate post-synaptic neurotransmission in hippocampal neurons. The molecular basis for this effect correlates with JRM-28-induced upregulation of the transcription of cAMP response element-binding protein(CREB), induction of its transcriptional activity, and subsequent stimulation of expressions of CREB-dependent plasticity-associated genes, such as those encoding N-methyl-D-aspartate (NMDA) receptor subunit NR2A and the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor subunit GluR1. Specifically, JRM-28 stimulated the NMDA- and AMPA-receptor-sensitive ionotropic calcium influx in hippocampal neurons. Interestingly, JRM-28 did not induce NMDA- and AMPA-sensitive calcium influx in hippocampal neurons once the expression of CREB was knocked down by creb siRNA, suggesting the critical role of CREB in JRM-28-mediated upregulation of synaptic plasticity. Finally, JRM-28 upregulated CREB mRNA, CREB-dependent plasticity-associated markers, and ionotropic calcium influx in iPSC-derived AD human neurons, indicating its therapeutic implications in the amelioration of AD pathologies.
Keywords: AMPA-sensitive glutamate receptor 1; HDAC inhibitor; Histone deacetylase 2; NMDA receptor subunit 2A; benzene; cAMP responsive element binding protein 2; dichloromethane; immunoblot; immunofluorescence; iodine; lithium diisopropylamide; meta-chloroperoxybenzoic acid; methanol; sodium acetate; tetrahydrofuran; triethyl amine.
Conflict of interest statement
S.B., C.G.G. and A.R. are employees of Simmaron Research Institute, other authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

