Mass Spectrometry-Based Workflow for the Identification and Quantification of Alternative and Canonical Proteins in Pancreatic Cancer Cells
- PMID: 39682715
- PMCID: PMC11640293
- DOI: 10.3390/cells13231966
Mass Spectrometry-Based Workflow for the Identification and Quantification of Alternative and Canonical Proteins in Pancreatic Cancer Cells
Abstract
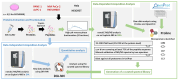
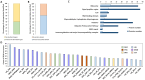
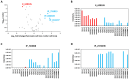
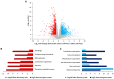
The identification of small proteins and proteins produced from unannotated open reading frames (called alternative proteins or AltProts) has changed our vision of the proteome and has attracted more and more attention from the scientific community. Despite several studies investigating particular AltProts in diseases and demonstrating their importance in such context, we are still missing data on their expression and functions in many pathologies. Among these, pancreatic ductal adenocarcinoma (PDAC) is a particularly relevant case to study alternative proteins. Indeed, late detection of this disease, notably due to the lack of reliable biomarkers of early-stage PDAC, and the fact that tumors rapidly develop resistance to most of the treatments used in the clinics warrant the exploration of new repertoires of molecules. In the present article, we aim to investigate the alternative proteome of pancreatic cancer cell lines as a first attempt to decipher the expression of AltProts in PDAC. Thanks to a combined data-dependent and data-independent acquisition mass spectrometry workflow, we were able to identify tryptic peptides matching 113 AltProts in a panel of 6 cell lines. In addition, we identified AltProts differentially expressed between pancreatic cancer cell lines and other cells (HeLa and HEK293T). Finally, mining the TCGA and Gtex databases showed that the corresponding transcripts encoding several AltProts we identified are differentially expressed between PDAC tumors and normal tissues and are correlated with the patient's survival.
Keywords: alternative proteins; data independent acquisition; microproteins; pancreatic ductal adenocarcinoma; proteomics; short open reading frame-encoded peptides.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

