Unveiling Smyd-2's Role in Cytoplasmic Nrf-2 Sequestration and Ferroptosis Induction in Hippocampal Neurons After Cerebral Ischemia/Reperfusion
- PMID: 39682718
- PMCID: PMC11639856
- DOI: 10.3390/cells13231969
Unveiling Smyd-2's Role in Cytoplasmic Nrf-2 Sequestration and Ferroptosis Induction in Hippocampal Neurons After Cerebral Ischemia/Reperfusion
Abstract
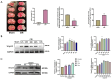
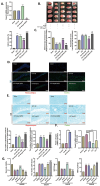
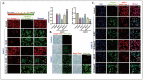
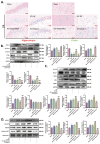
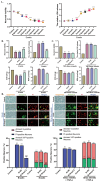
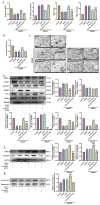
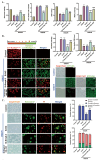
SET and MYND Domain-Containing 2 (Smyd-2), a specific protein lysine methyltransferase (PKMT), influences both histones and non-histones. Its role in cerebral ischemia/reperfusion (CIR), particularly in ferroptosis-a regulated form of cell death driven by lipid peroxidation-remains poorly understood. This study identifies the expression of Smyd-2 in the brain and investigates its relationship with neuronal programmed cell death (PCD). We specifically investigated how Smyd-2 regulates ferroptosis in CIR through its interaction with the Nuclear Factor Erythroid-2-related Factor-2 (Nrf-2)/Kelch-like ECH-associated protein (Keap-1) pathway. Smyd-2 knockout protects HT-22 cells from Erastin-induced ferroptosis but not TNF-α + Smac-mimetic-induced apoptosis/necroptosis. This neuroprotective effect of Smyd-2 knockout in HT-22 cells after Oxygen-Glucose Deprivation/Reperfusion (OGD/R) was reversed by Erastin. Smyd-2 knockout in HT-22 cells shows neuroprotection primarily via the Nuclear Factor Erythroid-2-related Factor-2 (Nrf-2)/Kelch-like ECH-associated protein (Keap-1) pathway, despite the concurrent upregulation of Smyd-2 and Nrf-2 observed in both the middle cerebral artery occlusion (MCAO) and OGD/R models. Interestingly, vivo experiments demonstrated that Smyd-2 knockout significantly reduced ferroptosis and lipid peroxidation in hippocampal neurons following CIR. Moreover, the Nrf-2 inhibitor ML-385 abolished the neuroprotective effects of Smyd-2 knockout, confirming the pivotal role of Nrf-2 in ferroptosis regulation. Cycloheximide (CHX) fails to reduce Nrf-2 expression in Smyd-2 knockout HT-22 cells. Smyd-2 knockout suppresses Nrf-2 lysine methylation, thereby promoting the Nrf-2/Keap-1 pathway without affecting the PKC-δ/Nrf-2 pathway. Conversely, Smyd-2 overexpression disrupts Nrf-2 nuclear translocation, exacerbating ferroptosis and oxidative stress, highlighting its dual regulatory role. This study underscores Smyd-2's potential for ischemic stroke treatment by disrupting the Smyd-2/Nrf-2-driven antioxidant capacity, leading to hippocampal neuronal ferroptosis. By clarifying the intricate interplay between ferroptosis and oxidative stress via the Nrf-2/Keap-1 pathway, our findings provide new insights into the molecular mechanisms of CIR and identify Smyd-2 as a promising therapeutic target.
Keywords: Nrf-2; Smyd-2; ferroptosis; hinge and latch; ischemic stroke; lipid peroxidation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

