CD38 Inhibitor 78c Attenuates Pro-Inflammatory Cytokine Expression and Osteoclastogenesis in Macrophages
- PMID: 39682719
- PMCID: PMC11640151
- DOI: 10.3390/cells13231971
CD38 Inhibitor 78c Attenuates Pro-Inflammatory Cytokine Expression and Osteoclastogenesis in Macrophages
Abstract
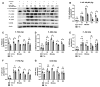
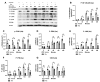
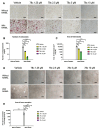
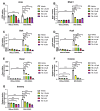
CD38, a nicotinamide adenine dinucleotide (NAD+) glycohydrolase, increases during infection or inflammation. Therefore, we aimed to evaluate the effects of a CD38 inhibitor (78c) on NAD+ levels, IL-1β, IL-6, TNF-α cytokine expressions, and osteoclastogenesis. The results show that treatment with 78c on murine BMMs dose-dependently reduced CD38, reversed the decline of NAD+, and inhibited IL-1β, IL-6, and TNF-α pro-inflammatory cytokine levels induced by oral pathogen Porphyromonas gingivalis (Pg) or Aggregatibacter actinomycetemcomitans (Aa) or by advanced glycation end products (AGEs). Additionally, treatment with 78c dose-dependently suppressed osteoclastogenesis and bone resorption induced by RANKL. Treatment with 78c suppressed CD38, nuclear factor kappa-B (NF-κB), phosphoinositide 3-kinase (PI3K), and mitogen-activated protein kinases (MAPKs) induced by Pg, Aa, or AGEs, and suppressed podosome components (PI3K, Pyk2, Src, F-actin, integrins, paxillin, and talin) induced by RANKL. These results from our studies support the finding that the inhibition of CD38 by 78c is a promising therapeutic strategy to treat inflammatory bone loss diseases. However, treatment with a CD38 shRNA only significantly reduced IL-1β, IL-6, and TNF-α pro-inflammatory cytokine levels induced by AGEs. Compared with controls, it had limited effects on cytokine levels induced by Pg or Aa. Treatment with the CD38 shRNA enhanced RANKL-induced osteoclastogenesis, suggesting that 78c has some off-target effects.
Keywords: CD38; NAD+; bone loss; cytokine; osteoclast; podosome.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Inhibition of CD38 by 78c Enhanced NAD+, Alleviated Inflammation, and Decreased Oxidative Stress in Old Murine Macrophages Induced by Oral Pathogens.Int J Mol Sci. 2025 Jun 26;26(13):6180. doi: 10.3390/ijms26136180. Int J Mol Sci. 2025. PMID: 40649955 Free PMC article.
-
FTY720 inhibited proinflammatory cytokine release and osteoclastogenesis induced by Aggregatibacter actinomycetemcomitans.Lipids Health Dis. 2015 Jul 4;14:66. doi: 10.1186/s12944-015-0057-7. Lipids Health Dis. 2015. PMID: 26138336 Free PMC article.
-
Sphingosine-1-Phosphate Receptor 2 Controls Podosome Components Induced by RANKL Affecting Osteoclastogenesis and Bone Resorption.Cells. 2019 Jan 1;8(1):17. doi: 10.3390/cells8010017. Cells. 2019. PMID: 30609675 Free PMC article.
-
Glycogen Synthase Kinase-3 Beta (GSK3β) as a Potential Drug Target in Regulating Osteoclastogenesis: An Updated Review on Current Evidence.Biomolecules. 2024 Apr 21;14(4):502. doi: 10.3390/biom14040502. Biomolecules. 2024. PMID: 38672518 Free PMC article. Review.
-
Docosahexaenoic Acid Inhibits Osteoclastogenesis via FFAR4-Mediated Regulation of Inflammatory Cytokines.Molecules. 2025 Jul 29;30(15):3180. doi: 10.3390/molecules30153180. Molecules. 2025. PMID: 40807354 Free PMC article. Review.
Cited by
-
Inhibition of CD38 by 78c Enhanced NAD+, Alleviated Inflammation, and Decreased Oxidative Stress in Old Murine Macrophages Induced by Oral Pathogens.Int J Mol Sci. 2025 Jun 26;26(13):6180. doi: 10.3390/ijms26136180. Int J Mol Sci. 2025. PMID: 40649955 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

