The Potential of a Novel Cold Atmospheric Plasma Jet as a Feasible Therapeutic Strategy for Gingivitis-A Cell-Based Study
- PMID: 39682721
- PMCID: PMC11640168
- DOI: 10.3390/cells13231970
The Potential of a Novel Cold Atmospheric Plasma Jet as a Feasible Therapeutic Strategy for Gingivitis-A Cell-Based Study
Abstract
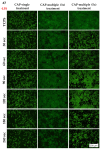
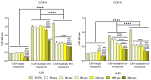
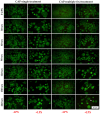
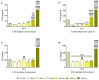
Due to its antimicrobial, anti-inflammatory and pro-healing properties, the application of cold atmospheric plasma (CAP) has emerged as a new and promising therapeutic strategy in various fields of medicine, including general medicine and dentistry. In this light, the aim of the present study was to investigate the effects of a homemade plasma jet on the cellular behaviour of two important cell types involved in gingivitis, namely gingival fibroblasts (HGF-1 cell line) and macrophages (RAW 264.7 cell line), by the direct application of CAP in different experimental conditions. The cellular behaviour of the HGF-1 cells was investigated in terms of viability/proliferation (LIVE/DEAD and CCK-8 assays), morphological features (immunofluorescent staining of the actin cytoskeleton) and fibronectin expression (immunocytochemical staining of the fibronectin network), while the macrophages' response was evaluated through the assessment of the cellular survival/proliferation rate (LIVE/DEAD and CCK-8 assays), morphological behaviour (immunofluorescent staining of the actin cytoskeleton) and inflammatory activity (pro-inflammatory cytokine secretion profile (ELISA assay) and foreign body giant cells (FBGCs) formation (immunofluorescent staining of the actin cytoskeleton and multinuclearity index determination)). The in vitro biological assessment revealed an upward trend dependent on treatment time and number of CAP applications, in terms of fibroblasts proliferation (p < 0.0001) and fibronectin expression (p < 0.0001). On the other hand, the macrophages exposed to five consecutive CAP applications for longer treatment times (over 120 s) exhibited a strong pro-inflammatory activity, as evinced by their altered morphology, pro-inflammatory cytokine profile (p < 0.0001) and FBGCs formation. Overall, our results demonstrate that CAP exposure, when used with appropriate operating parameters, has a beneficial effect on the cellular response of HGF-1 and RAW 264.7 cells, thus paving the way for further in vitro and in vivo investigations that will allow the translation of CAP treatment from research to clinic as an alternative therapy for gingivitis.
Keywords: cold atmospheric plasma; gingival fibroblasts; gingivitis; inflammation; macrophages; wound healing.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Non-thermal atmospheric pressure plasma increased mRNA expression of growth factors in human gingival fibroblasts.Clin Oral Investig. 2016 Sep;20(7):1801-8. doi: 10.1007/s00784-015-1668-0. Epub 2015 Nov 27. Clin Oral Investig. 2016. PMID: 26612399
-
Safety and bactericidal efficacy of cold atmospheric plasma generated by a flexible surface Dielectric Barrier Discharge device against Pseudomonas aeruginosa in vitro and in vivo.Ann Clin Microbiol Antimicrob. 2020 Aug 19;19(1):37. doi: 10.1186/s12941-020-00381-z. Ann Clin Microbiol Antimicrob. 2020. PMID: 32814573 Free PMC article.
-
Cold atmospheric plasma (CAP) changes gene expression of key molecules of the wound healing machinery and improves wound healing in vitro and in vivo.PLoS One. 2013 Nov 12;8(11):e79325. doi: 10.1371/journal.pone.0079325. eCollection 2013. PLoS One. 2013. PMID: 24265766 Free PMC article.
-
The therapeutic perspective of cold atmospheric plasma in periodontal disease.Oral Dis. 2024 Apr;30(3):938-948. doi: 10.1111/odi.14547. Epub 2023 Mar 29. Oral Dis. 2024. PMID: 36825384 Review.
-
Effectiveness of cold plasma application in oral wound healing process: A scoping review.Oral Dis. 2024 Nov;30(8):5062-5081. doi: 10.1111/odi.15119. Epub 2024 Sep 3. Oral Dis. 2024. PMID: 39224064
References
-
- Gaton J.G., Armitage G., Berglundh T., Chapple I.L.C., Jepsen S., Kornman K.S., Mealey B.L., Papapanou P.N., Sanz M. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Periodontol. 2018;89:S1–S8. - PubMed
-
- Daleh M.H.A., Dias D.R., Kumar P. The economic and societal impact of periodontal and peri-implant diseases. Periodontol. 2000. 2024 Online ahead of print . - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

