Motile Cilia in Female and Male Reproductive Tracts and Fertility
- PMID: 39682722
- PMCID: PMC11639810
- DOI: 10.3390/cells13231974
Motile Cilia in Female and Male Reproductive Tracts and Fertility
Abstract
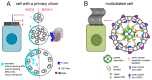
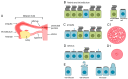
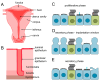
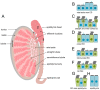
Motile cilia are evolutionarily conserved organelles. In humans, multiciliated cells (MCCs), assembling several hundred motile cilia on their apical surface, are components of the monolayer epithelia lining lower and upper airways, brain ventricles, and parts of the reproductive tracts, the fallopian tube and uterus in females, and efferent ductules in males. The coordinated beating of cilia generates a force that enables a shift of the tubular fluid, particles, or cells along the surface of the ciliated epithelia. Uncoordinated or altered cilia motion or cilia immotility may result in subfertility or even infertility. Here, we summarize the current knowledge regarding the localization and function of MCCs in the human reproductive tracts, discuss how cilia and cilia beating-generated fluid flow directly and indirectly contribute to the processes in these organs, and how lack or improper functioning of cilia influence human fertility.
Keywords: cilia; ectopic pregnancy; efferent ductules; endometrium; fallopian tube; flagella; infertility; uterus.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

