Herpes Simplex Virus 1 Infection of Human Brain Organoids and Pancreatic Stem Cell-Islets Drives Organoid-Specific Transcripts Associated with Alzheimer's Disease and Autoimmune Diseases
- PMID: 39682726
- PMCID: PMC11640215
- DOI: 10.3390/cells13231978
Herpes Simplex Virus 1 Infection of Human Brain Organoids and Pancreatic Stem Cell-Islets Drives Organoid-Specific Transcripts Associated with Alzheimer's Disease and Autoimmune Diseases
Abstract
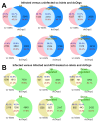
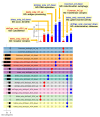
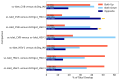
Viral infections leading to inflammation have been implicated in several common diseases, such as Alzheimer's disease (AD) and type 1 diabetes (T1D). Of note, herpes simplex virus 1 (HSV-1) has been reported to be associated with AD. We sought to identify the transcriptomic changes due to HSV-1 infection and anti-viral drug (acyclovir, ACV) treatment of HSV-1 infection in dissociated cells from human cerebral organoids (dcOrgs) versus stem cell-derived pancreatic islets (sc-islets) to gain potential biological insights into the relevance of HSV-1-induced inflammation in AD and T1D. We observed that differentially expressed genes (DEGs) in HSV-1-infected sc-islets were enriched for genes associated with several autoimmune diseases, most significantly, T1D, but also rheumatoid arthritis, psoriasis, Crohn's disease, and multiple sclerosis, whereas DEGs in HSV-1-infected dcOrgs were exclusively enriched for genes associated with AD. The ACV treatment of sc-islets was not as effective in rescuing transcript perturbations of autoimmune disease-associated genes. Finally, we identified gene ontology categories that were enriched for DEGs that were in common across, or unique to, viral treatment of dcOrgs and sc-islets, such as categories involved in the transferase complex, mitochondrial, and autophagy function. In addition, we compared transcriptomic signatures from HSV-1-infected sc-islets with sc-islets that were infected with the coxsackie B virus (CVB) that had been associated with T1D pathogenesis. Collectively, this study provides tissue-specific insights into the molecular effects of inflammation in AD and T1D.
Keywords: Alzheimer’s disease; acyclovir; autoimmune diseases; cerebral organoids; herpes simplex virus 1; innate immune; neurodegenerative diseases; stem cell islets; type 1 diabetes.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Eimer W.A., Vijaya Kumar D.K., Navalpur Shanmugam N.K., Rodriguez A.S., Mitchell T., Washicosky K.J., Gyorgy B., Breakefield X.O., Tanzi R.E., Moir R.D. Alzheimer’s Disease-Associated beta-Amyloid Is Rapidly Seeded by Herpesviridae to Protect against Brain Infection. Neuron. 2018;99:56–63 e3. doi: 10.1016/j.neuron.2018.06.030. - DOI - PMC - PubMed
-
- Olson M.N., Dawes P., Murray L.F., Barton N.J., Sundstrom J., Orszulak A.R., Chigas S.M., Tran K., Aylward A.J., Caliandro M.F., et al. Development of a high-throughput, quantitative platform using human cerebral organoids to study virus-induced neuroinflammation in Alzheimer’s disease. bioRxiv. 2024 doi: 10.1101/2024.03.21.585957. - DOI
Publication types
MeSH terms
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

