Class B Scavenger Receptor CD36 as a Potential Therapeutic Target in Inflammation Induced by Danger-Associated Molecular Patterns
- PMID: 39682740
- PMCID: PMC11640246
- DOI: 10.3390/cells13231992
Class B Scavenger Receptor CD36 as a Potential Therapeutic Target in Inflammation Induced by Danger-Associated Molecular Patterns
Abstract
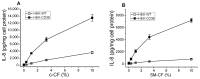
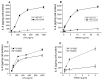
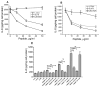
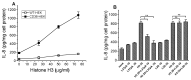
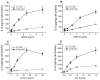
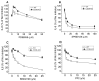
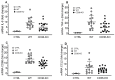
The class B scavenger receptor CD36 is known to bind and mediate the transport of lipid-related ligands and it functions as a pattern recognition receptor (PRR) for a variety of pathogens, including bacteria and viruses. In this study, we assessed CD36's role as a PRR mediating pro-inflammatory effects of several known Danger-Associated Molecular Patterns (DAMPs) used either as a single preparation or as a combination of DAMPs in the form of total cell/skeletal muscle tissue lysates. Our data demonstrated that multiple DAMPs, including HMGB1, HSPs, histone H3, SAA, and oxPAPC, as well as cell/tissue lysate preparations, induced substantially higher (~7-10-fold) IL-8 cytokine responses in HEK293 cells overexpressing CD36 compared to control WT cells. At the same time, DAMP-induced secretion of IL-6 in bone marrow-derived macrophages (BMDM) from CD36-/- mice was markedly (~2-3 times) reduced, as compared to macrophages from normal mice. Synthetic amphipathic helical peptides (SAHPs), known CD36 ligands, efficiently blocked CD36-dependent inflammatory responses induced by both cell and tissue lysates, HMGB1 and histone H3 in CD36+ cells. IP injection of total cellular lysate preparation induced inflammatory responses that were assessed by the expression of liver and lung pro-inflammatory markers, including IL-6, TNF-α, CD68, and CXCL1, and was reduced by ~50% in CD36-deficient mice compared to normal mice. Our findings demonstrate that CD36 is a PRR contributing to the innate immune response via mediating DAMP-induced inflammatory signaling and highlight the importance of this receptor as a potential therapeutic target in DAMP-associated inflammatory conditions.
Keywords: CD36; DAMPs; HMGB1; SAA; heat shock proteins; histones; inflammatory markers; oxPAPC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Baranova I.N., Bocharov A.V., Vishnyakova T.G., Kurlander R., Chen Z., Fu D., Arias I.M., Csako G., Patterson A.P., Eggerman T.L. CD36 is a novel serum amyloid A (SAA) receptor mediating SAA binding and SAA-induced signaling in human and rodent cells. J. Biol. Chem. 2010;285:8492–8506. doi: 10.1074/jbc.M109.007526. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

