Differential Infiltration of Key Immune T-Cell Populations Across Malignancies Varying by Immunogenic Potential and the Likelihood of Response to Immunotherapy
- PMID: 39682743
- PMCID: PMC11640164
- DOI: 10.3390/cells13231993
Differential Infiltration of Key Immune T-Cell Populations Across Malignancies Varying by Immunogenic Potential and the Likelihood of Response to Immunotherapy
Abstract
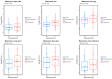
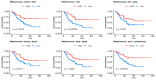
Background: Solid tumors vary by the immunogenic potential of the tumor microenvironment (TME) and the likelihood of response to immunotherapy. The emerging literature has identified key immune cell populations that significantly impact immune activation or suppression within the TME. This study investigated candidate T-cell populations and their differential infiltration within different tumor types as estimated from mRNA co-expression levels of the corresponding cellular markers. Methods: We analyzed the mRNA co-expression levels of cellular biomarkers that define stem-like tumor-infiltrating lymphocytes (TILs), tissue-resident memory T-cells (TRM), early dysfunctional T-cells, late dysfunctional T-cells, activated-potentially anti-tumor (APA) T-cells and Butyrophilin 3A (BTN3A) isoforms, utilizing clinical and transcriptomic data from 1892 patients diagnosed with melanoma, bladder, ovarian, or pancreatic carcinomas. Real-world data were collected under the Total Cancer Care Protocol and the Avatar® project (NCT03977402) across 18 cancer centers. Furthermore, we compared the survival outcomes following immune checkpoint inhibitors (ICIs) based on immune cell gene expression. Results: In melanoma and bladder cancer, the estimated infiltration of APA T-cells differed significantly (p = 4.67 × 10-12 and p = 5.80 × 10-12, respectively) compared to ovarian and pancreatic cancers. Ovarian cancer had lower TRM T-cell infiltration than melanoma, bladder, and pancreatic (p = 2.23 × 10-8, 3.86 × 10-28, and 7.85 × 10-9, respectively). Similar trends were noted with stem-like, early, and late dysfunctional T-cells. Melanoma and ovarian expressed BTN3A isoforms more than other malignancies. Higher densities of stem-like TILs; TRM, early and late dysfunctional T-cells; APA T-cells; and BTN3A isoforms were associated with increased survival in melanoma (p = 0.0075, 0.00059, 0.013, 0.005, 0.0016, and 0.041, respectively). The TRM gene signature was a moderate predictor of survival in the melanoma cohort (AUROC = 0.65), with similar findings in testing independent public datasets of ICI-treated patients with melanoma (AUROC 0.61-0.64). Conclusions: Key cellular elements related to immune activation are more heavily infiltrated within ICI-responsive versus non-responsive malignancies, supporting a central role in anti-tumor immunity. In melanoma patients treated with ICIs, higher densities of stem-like TILs, TRM T-cells, early dysfunctional T-cells, late dysfunctional T-cells, APA T-cells, and BTN3A isoforms were associated with improved survival.
Keywords: RNA expression; T-cell; bladder cancer; immune cell infiltration; immune response; melanoma; ovarian cancer; pancreatic cancer.
Conflict of interest statement
I.E., S.C., M.L.C., J.C., W.S.D., G.J.W., D.S., B.S., J.M., S.G., A.R., C.S., A.R.N., and P.H. declare no conflicts of interest. M.M. declares contracted research grants with Merck, Taiho, and the National Comprehensive Cancer Network. IP declares Program Leader (1.2 cal months) for NIH/NCI Cancer Center Support Grant (P30CA016056); consulting with Nouscom, Iovance, Nektar, and Regeneron; and stock owner with Ideaya, Inc. H.C. declares advisory board/consultant: Best Doctors/Teladoc, Orbus Therapeutics, Bristol Meyers Squibb, Regeneron, Novocure, and PPD/Chimerix and research funding (site PI/institutional contract): Orbus, GCAR, Array BioPharma, Karyopharm Therapeutics, Nuvation Bio, Bayer, Bristol Meyer Squib, and Sumitomo Dainippon Pharma. To date, S.M.A. is a member of ASCO COI. I.M.E.N. is on the scientific advisory board of Endectra. L.L. acts as deputy editor for the Journal of Medical Physics and receives funding from NIH and DoD. J.R.C.G has stock options in Compass Therapeutics, Anixa Biosciences and Alloy Therapeutics; receives consulting fees from Alloy Therapeutics; has intellectual property with Compass Therapeutics and Anixa Biosciences; receives licensing fees from Anixa Biosciences; and is co-founder of Cellepus Therapeutics. A.A.T. declares contracted research grants with their institution from Bristol Myers Squib, Genentech-Roche, Regeneron, Sanofi-Genzyme, Nektar, Clinigen, Merck, Acrotech, Pfizer, Checkmate, OncoSec, Scholar Rock, InflaRx GmbH, Agenus and personal consultant/advisory board fees from Bristol Myers Squibb, Merck, Easai, Instil Bio, Clinigin, Regeneron, Sanofi-Genzyme, Novartis, Partner Therapeutics, Genentech/Roche, BioNTech, Concert AI, AstraZeneca outside the submitted work.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

