Therapeutic Exploitation of Neuroendocrine Transdifferentiation Drivers in Prostate Cancer
- PMID: 39682746
- PMCID: PMC11639977
- DOI: 10.3390/cells13231999
Therapeutic Exploitation of Neuroendocrine Transdifferentiation Drivers in Prostate Cancer
Abstract
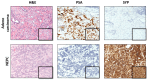
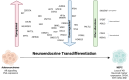
Neuroendocrine prostate cancer (NEPC), an aggressive and lethal subtype of prostate cancer (PCa), often arises as a resistance mechanism in patients undergoing hormone therapy for prostate adenocarcinoma. NEPC is associated with a significantly poor prognosis and shorter overall survival compared to conventional prostate adenocarcinoma due to its aggressive nature and limited response to standard of care therapies. This transdifferentiation, or lineage reprogramming, to NEPC is characterised by the loss of androgen receptor (AR) and prostate-specific antigen (PSA) expression, and the upregulation of neuroendocrine (NE) biomarkers such as neuron-specific enolase (NSE), chromogranin-A (CHGA), synaptophysin (SYP), and neural cell adhesion molecule 1 (NCAM1/CD56), which are critical for NEPC diagnosis. The loss of AR expression culminates in resistance to standard of care PCa therapies, such as androgen-deprivation therapy (ADT) which target the AR signalling axis. This review explores the drivers of NE transdifferentiation. Key genetic alterations, including those in the tumour suppressor genes RB1, TP53, and PTEN, and changes in epigenetic regulators, particularly involving EZH2 and cell-fate-determining transcription factors (TFs) such as SOX2, play significant roles in promoting NE transdifferentiation and facilitate the lineage switch from prostate adenocarcinoma to NEPC. The recent identification of several other key novel drivers of NE transdifferentiation, including MYCN, ASCL1, BRN2, ONECUT2, and FOXA2, further elucidates the complex regulatory networks and pathways involved in this process. We suggest that, given the multifactorial nature of NEPC, novel therapeutic strategies that combine multiple modalities are essential to overcome therapeutic resistance and improve patient outcomes.
Keywords: cancer; lineage plasticity; neuroendocrine; prostate; transdifferentiation.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

