Novel mTORC2/HSPB4 Interaction: Role and Regulation of HSPB4 T148 Phosphorylation
- PMID: 39682748
- PMCID: PMC11640050
- DOI: 10.3390/cells13232000
Novel mTORC2/HSPB4 Interaction: Role and Regulation of HSPB4 T148 Phosphorylation
Abstract
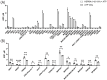
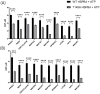
HSPB4 and HSPB5 (α-crystallins) have shown increasing promise as neuroprotective agents, demonstrating several anti-apoptotic and protective roles in disorders such as multiple sclerosis and diabetic retinopathy. HSPs are highly regulated by post-translational modification, including deamidation, glycosylation, and phosphorylation. Among them, T148 phosphorylation has been shown to regulate the structural and functional characteristics of HSPB4 and underlie, in part, its neuroprotective capacity. We recently demonstrated that this phosphorylation is reduced in retinal tissues from patients with diabetic retinopathy, raising the question of its regulation during diseases. The kinase(s) responsible for regulating this phosphorylation, however, have yet to be identified. To this end, we employed a multi-tier strategy utilizing in vitro kinome profiling, bioinformatics, and chemoproteomics to predict and discover the kinases capable of phosphorylating T148. Several kinases were identified as being capable of specifically phosphorylating T148 in vitro, and further analysis highlighted mTORC2 as a particularly strong candidate. Altogether, our data demonstrate that the HSPB4-mTORC2 interaction is multi-faceted. Our data support the role of mTORC2 as a specific kinase phosphorylating HSPB4 at T148, but also provide evidence that the HSPB4 chaperone function further strengthens the interaction. This study addresses a critical gap in our understanding of the regulatory underpinnings of T148 phosphorylation-mediated neuroprotection.
Keywords: HSPB4; chaperone; kinase; neuroprotection; phosphorylation; sHSP; αA-crystallin.
Conflict of interest statement
Fort has intellectual property interests and is listed as an inventor on a patent for the use of α-crystallins as a retinal neuroprotective treatment. All the other authors have no conflicts of interest to disclose.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

