Riluzole Reverses Blood-Testis Barrier Loss to Rescue Chemotherapy-Induced Male Infertility by Binding to TRPC
- PMID: 39682764
- PMCID: PMC11640501
- DOI: 10.3390/cells13232016
Riluzole Reverses Blood-Testis Barrier Loss to Rescue Chemotherapy-Induced Male Infertility by Binding to TRPC
Abstract
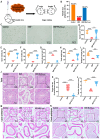
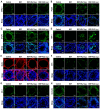
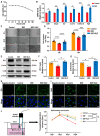
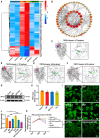
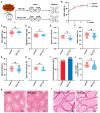
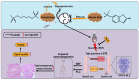
Cancer treatments, including cytotoxic therapy, often result in male infertility, necessitating the development of safe and effective strategies to preserve male reproductive potential during chemotherapy. Notably, our study uncovers the potential of repurposing riluzole, an FDA-approved drug for amyotrophic lateral sclerosis (ALS), in enhancing spermatogenesis. Hence, this research aims to explore the feasibility of utilizing riluzole to alleviate male infertility induced by busulfan (BSF), a commonly used chemotherapy drug. We established a BSF-induced oligospermia model in 4-week-old male mice and found that riluzole could effectively counter the detrimental effects of BSF on sperm production in mice with oligospermia. By restoring blood-testis barrier (BTB) functionality, riluzole improves sperm quality and reduces testicular atrophy. Through transcriptomic and molecular docking analyses, we identify transient receptor potential canonical subfamily member 5 (TRPC5) as a potential target for riluzole-mediated regulation of blood-testis barrier function. These findings propose riluzole as a promising therapeutic option for chemotherapy-induced male infertility, thereby addressing the fertility challenges associated with cancer treatments. Moreover, repurposing riluzole could streamline the drug development process, providing a cost-effective approach with reduced risk compared to developing entirely new drugs.
Keywords: Sertoli cells; TRPC5; blood–testis barrier; male infertility; riluzole; spermatogenesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Perrone P., Lettieri G., Marinaro C., Longo V., Capone S., Forleo A., Pappalardo S., Montano L., Piscopo M. Molecular Alterations and Severe Abnormalities in Spermatozoa of Young Men Living in the “Valley of Sacco River” (Latium, Italy): A Preliminary Study. Int. J. Environ. Res. Public Health. 2022;19:11023. doi: 10.3390/ijerph191711023. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

