Senescence and Stress Signaling Pathways in Corneal Cells After Nitrogen Mustard Injury
- PMID: 39682768
- PMCID: PMC11640117
- DOI: 10.3390/cells13232021
Senescence and Stress Signaling Pathways in Corneal Cells After Nitrogen Mustard Injury
Abstract
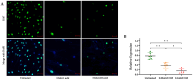
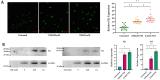
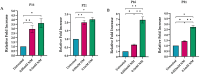
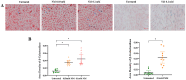
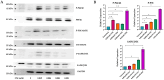
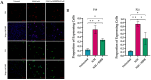
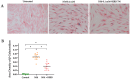
Mustard gas keratopathy (MGK), a complication of exposure to sulfur mustard, is a blinding ocular surface disease involving key cellular pathways, including apoptosis, oxidative stress, and inflammation. Recent studies indicate that cellular senescence contributes to the pathophysiology of mustard gas toxicity. This study aimed to assess senescence and stress-related pathways-particularly mitogen-activated protein kinase (MAPK) signaling-in nitrogen mustard (NM)-induced corneal injury. In vitro, primary human corneal epithelial (P-HCECs), primary human corneal mesenchymal stromal cells (hcMSCs), and human corneal-limbal epithelial cell (HCLE) lines were exposed to varying concentrations of NM. The results demonstrated a dose-dependent increase in cellular senescence, characterized by reduced Ki67 expression, elevated p16, and p21 mRNA levels, as well as activation of the MAPK pathway activation. Treatment with a selective p38-MAPK inhibitor significantly reduced senescence markers and improved cell proliferation following exposure to NM. Overall, these studies indicate that NM exposure triggers cellular senescence and stress-related MAPK signaling, while p38-MAPK inhibition mitigates these effects, suggesting a potential therapeutic strategy.
Keywords: BIRB796; MAPK; MAPK inhibitor; cornea; mustard; mustard keratopathy; nitrogen mustard; ocular surface; senescence.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Soleimani M., Momenaei B., Baradaran-Rafii A., Cheraqpour K., An S., Ashraf M.J., Abedi F., Javadi M.A., Djalilian A.R. Mustard Gas-Induced Ocular Surface Disorders: An Update on the Pathogenesis, Clinical Manifestations, and Management. Cornea. 2023;42:776–786. doi: 10.1097/ICO.0000000000003182. - DOI - PMC - PubMed
-
- Mishra N., Kant R., Kandhari K., Ammar D.A., Tewari-Singh N., Pantcheva M.B., Petrash J.M., Agarwal C., Agarwal R. Nitrogen Mustard-Induced Ex Vivo Human Cornea Injury Model and Therapeutic Intervention by Dexamethasone. J. Pharmacol. Exp. Ther. 2024;388:484–494. doi: 10.1124/jpet.123.001760. - DOI - PMC - PubMed
-
- Alemi H., Dehghani S., Musayeva A., Nadari A., Narimatsu A., Sharifi S., Forouzanfar K., Wang S., Dohlman T.H., Yin J., et al. Insights into mustard gas keratopathy: Characterizing corneal layer-specific changes in mice exposed to nitrogen mustard. Exp. Eye Res. 2023;236:109495. doi: 10.1016/j.exer.2023.109495. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 EY001792/EY/NEI NIH HHS/United States
- UH3 EY031809/EY/NEI NIH HHS/United States
- R01EY035221 (A.R.D.)/NEI/NIH
- the Unrestricted Grant to the department and Physician-Scientist Award/Research to Prevent Blindness
- the Vision Research Program - Congressionally Directed Medical Research Program VR170180/Department of Defense
LinkOut - more resources
Full Text Sources

