Clinical Potential of Novel Microbial Therapeutic LP51 Based on Xerosis-Microbiome Index
- PMID: 39682776
- PMCID: PMC11639849
- DOI: 10.3390/cells13232029
Clinical Potential of Novel Microbial Therapeutic LP51 Based on Xerosis-Microbiome Index
Abstract
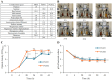
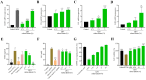
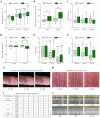
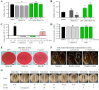
Xerosis, characterized by dry, rough skin, causes discomfort and aesthetic concerns, necessitating effective treatment. Traditional treatments often show limited efficacy, prompting the need for innovative therapies. This study highlights the efficacy of microbiome therapeutic LP51, derived from a healthy vaginal microbiome, in improving xerosis. A double-blind clinical trial involving 43 subjects with dry inner arm skin compared the effects of a 2.9% LP51 extract formulation to a placebo over 4 weeks. The LP51 group exhibited a significant increase in stratum corneum hydration (10.0 A.U.) compared to the placebo group (4.8 A.U.) and a 21.4% decrease in transepidermal water loss (TEWL), whereas the placebo group showed no significant change. LP51 also demonstrated benefits in enhancing skin hydration, improving the skin barrier, and exhibited anti-atopic, anti-inflammatory, and antioxidant properties. Safety was confirmed through in vitro cytotoxicity tests. These effects are attributed to the microbiome-safe component in LP51 and its role in improving xerosis, reflected by an increase in the xerosis-microbiome index, defined by the Firmicutes/Actinobacteria ratio. These findings position microbiome therapeutic LP51 as a promising novel treatment for xerosis.
Keywords: Firmicutes/Actinobacteria ratio; microbiome therapeutic LP51; vaginal microbiota; xerosis; xerosis-microbiome index.
Conflict of interest statement
H.-Y.S., S.K., H.S., J.H.H., J.-W.K., S.g.Y., J.H.K., Y.G.C. were employed by the company LABIO, Inc. and have filed a patent related to this research (Application number: 10-2023-0195000) The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Walters K.A., Roberts M.S. Dermatological and Transdermal Formulations. CRC Press; Boca Raton, FL, USA: 2002. The structure and function of skin; pp. 19–58.
-
- Archer C.B. Rook’s Textbook of Dermatology. Wiley-Blackwell Publishing; Hoboken, NJ, USA: 2010. Functions of the skin; pp. 1–11.
-
- Kamakshi R. Fairness via formulations: A review of cosmetic skin-lightening ingredients. J. Cosmet. Sci. 2012;63:43–54. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

