An Integrative Analysis of the Transcriptome and Proteome of Rice Grain Chalkiness Formation Under High Temperature
- PMID: 39683102
- PMCID: PMC11644674
- DOI: 10.3390/plants13233309
An Integrative Analysis of the Transcriptome and Proteome of Rice Grain Chalkiness Formation Under High Temperature
Abstract
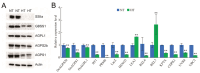
Exposure to high temperatures can impair the grain-filling process in rice (Oryza sativa L.), potentially leading to the formation of chalky endosperm, but the molecular regulation mechanism remains largely elusive. Here, we reported that high-temperature (HT) stress (day/night, 35 °C/30 °C) reduces both the grain-filling rate and grain weight of Ningjing 1 variety compared to normal temperatures (NT, day/night, 28 °C/23 °C). Grains under HT stress exhibited an opaque, milky-white appearance, alongside significant alterations in starch physicochemical properties. An integrated transcriptomic analysis of grains under HT revealed up-regulation of genes related to defense mechanisms and oxidoreductase activity, while genes involved in sucrose and starch synthesis were down-regulated, and α-amylase genes were up-regulated. Proteomic analysis of grains under HT echoed this pattern. These results demonstrate that high temperature during the grain-filling stage significantly increases rice chalkiness by down-regulating genes related to sucrose and starch synthesis, while up-regulating those involved in starch degradation.
Keywords: high temperature; proteome; rice quality; starch biosynthesis; transcriptome.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
High Temperature-Induced Expression of Rice α-Amylases in Developing Endosperm Produces Chalky Grains.Front Plant Sci. 2017 Dec 6;8:2089. doi: 10.3389/fpls.2017.02089. eCollection 2017. Front Plant Sci. 2017. PMID: 29270189 Free PMC article.
-
Proteomic and Glycomic Characterization of Rice Chalky Grains Produced Under Moderate and High-temperature Conditions in Field System.Rice (N Y). 2016 Dec;9(1):26. doi: 10.1186/s12284-016-0100-y. Epub 2016 May 31. Rice (N Y). 2016. PMID: 27246013 Free PMC article.
-
Transcriptome analysis of grain-filling caryopses reveals involvement of multiple regulatory pathways in chalky grain formation in rice.BMC Genomics. 2010 Dec 30;11:730. doi: 10.1186/1471-2164-11-730. BMC Genomics. 2010. PMID: 21192807 Free PMC article.
-
Relationship between High Temperature and Formation of Chalkiness and Their Effects on Quality of Rice.Biomed Res Int. 2018 Apr 1;2018:1653721. doi: 10.1155/2018/1653721. eCollection 2018. Biomed Res Int. 2018. PMID: 30065932 Free PMC article. Review.
-
[Current status and strategies for improvement of rice grain chalkiness].Yi Chuan. 2009 Jun;31(6):563-72. doi: 10.3724/sp.j.1005.2009.00563. Yi Chuan. 2009. PMID: 19586854 Review. Chinese.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

