Antiangiogenic Potential of Pomegranate Extracts
- PMID: 39683144
- PMCID: PMC11644541
- DOI: 10.3390/plants13233350
Antiangiogenic Potential of Pomegranate Extracts
Abstract
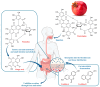
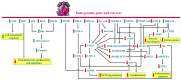
Pomegranate (Punica granatum L.) has long been recognised for its rich antioxidant profile and potential health benefits. Recent research has expanded its therapeutic potential to include antiangiogenic properties, which are crucial for inhibiting the growth of tumours and other pathological conditions involving aberrant blood vessel formation. This review consolidates current findings on the antiangiogenic effects of pomegranate extracts. We explore the impact of pomegranate polyphenols, including ellagic acid, punicalagin, anthocyanins, punicic acid and bioactive polysaccharides on key angiogenesis-related pathways and endothelial cell function. Emphasis is placed on the effects of these extracts as phytocomplexes rather than isolated compounds. Additionally, we discuss the use of pomegranate by-products, such as peels and seeds, in the preparation of extracts within a green chemistry and circular economy framework, highlighting their value in enhancing extract efficacy and sustainability. By primarily reviewing in vitro and in vivo preclinical studies, we assess how these extracts modulate angiogenesis across various disease models and explore their potential as adjunctive therapies for cancer and other angiogenesis-driven disorders. This review also identifies existing knowledge gaps and proposes future research directions to fully elucidate the clinical utility of pomegranate extracts in therapeutic applications.
Keywords: Punica granatum L.; VEGF; angiogenesis; cancer; ellagic acid; ellagitannins; extraction methods; punicalagin; punicic acid; supplement.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Davidescu L., Precup A.I., Fodor R., Ilias T.I. Investigating Mechanisms and Causes Related to Angiogenesis: A Review. Arch. Pharm. Pract. 2024;15:47–52. doi: 10.51847/QO4iMf1QmE. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

