Vitamin D Supplementation for Treating Atopic Dermatitis in Children and Adults: A Systematic Review and Meta-Analysis
- PMID: 39683522
- PMCID: PMC11644640
- DOI: 10.3390/nu16234128
Vitamin D Supplementation for Treating Atopic Dermatitis in Children and Adults: A Systematic Review and Meta-Analysis
Abstract
Background: Atopic dermatitis (AD) is a chronic inflammatory skin disease affecting up to 20% of children and 10% of adults worldwide. Current research suggests a correlation between serum vitamin D level and AD severity and that vitamin D supplementation could have a potential therapeutic effect on AD.
Objectives: To conduct a systematic review and meta-analysis of studies of vitamin D supplementation for disease improvement in children and adults with AD.
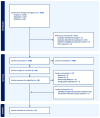
Methods: PubMed, EMBASE, and Cochrane were searched from 19 April to 20 April 2024. We included randomized controlled trials (RCTs) of patients with AD comparing an intervention group with a control group. The risk of bias of the selected studies was assessed using the Cochrane risk-of-bias tool for randomized trials. All analyses were conducted in R (v4.1.2; R Core Team 2021).
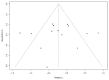
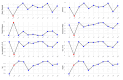
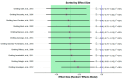
Results: Eleven RCTs with 686 participants were included. The included trials had measured AD severity by using the SCOring Atopic Dermatitis (SCORAD) or the Eczema Area and Severity Index (EASI). Vitamin D supplementation significantly reduced AD severity compared with the control group (standardized mean difference = -0.41, 95% CI: -0.67 to -0.16, I2 = 58%, p < 0.01).
Conclusions: Vitamin D supplementation reduces AD severity in children and adults. Larger-scale and longer-term studies are still needed to confirm this conclusion. This study has been registered on PROSPERO (CRD42024535014).
Keywords: SCORAD index; adults; atopic dermatitis; children; efficacy; human health; meta-analysis; systematic review; vitamin D.
Conflict of interest statement
Simon Francis Thomsen: With no relation to the present protocol, Thomsen has been a speaker or has served on advisory boards for Sanofi, AbbVie, LEO Pharma, Pfizer, Eli Lilly, Novartis, UCB Pharma, Union Therapeutics, Almirall, and Janssen Pharmaceuticals and has received research support from Sanofi, AbbVie, LEO Pharma, Novartis, UCB Pharma, and Janssen Pharmaceuticals. Howraman Meteran reports receiving honoraria for lectures and advisory board meetings from GSK, Teva, AstraZeneca, Novartis, Sanofi-Aventis, Airsonett AB, and ALK-Abelló not related to this study within the past 5 years. Howraman Meteran has received a research grant from ALK-Abelló A/S outside this study. The remaining authors have no conflicts of interest to report.
Figures







References
-
- Odhiambo J.A., Williams H.C., Clayton T.O., Robertson C.F., Asher M.I., ISAAC Phase Three Study Group Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J. Allergy Clin. Immunol. 2009;124:1251–1258.e23. - PubMed
-
- Silverberg J.I., Hanifin J.M. Adult eczema prevalence and associations with asthma and other health and demographic factors: A US population-based study. J. Allergy Clin. Immunol. 2013;132:1132–1138. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

