Influence of CReatine Supplementation on mUScle Mass and Strength After Stroke (ICaRUS Stroke Trial): A Randomized Controlled Trial
- PMID: 39683542
- PMCID: PMC11643803
- DOI: 10.3390/nu16234148
Influence of CReatine Supplementation on mUScle Mass and Strength After Stroke (ICaRUS Stroke Trial): A Randomized Controlled Trial
Abstract
Background/objectives: The acute phase of stroke is marked by inflammation and mobility changes that can compromise nutritional status. This study was a randomized, double-blind, placebo-controlled trial evaluating the effectiveness of creatine supplementation for older people during seven days of hospitalization for stroke compared to usual care.
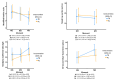
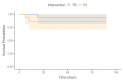
Method: The primary outcome measures were changes in functional capacity, strength, muscle mass, and muscle degradation. The secondary outcomes were changes in serum biomarkers related to inflammation, fibrosis, anabolism, and muscle synthesis. In addition, a follow-up 90 days after the stroke verified functional capacity, strength, quality of life, and mortality. Following admission for an acute stroke, participants received either creatine (10 g) or a visually identical placebo (10 g) orally twice daily. Both groups received supplementation with protein to achieve the goal of 1.5 g of protein/kg of body weight/day and underwent daily mobility training during seven days of hospitalization.
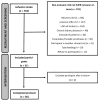
Results: Thirty older people were included in two similar groups concerning baseline attributes (15-treatment/15-placebo).
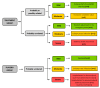
Conclusions: Creatine supplementation did not influence functional capacity, strength, or muscle mass during the first 7 days or outcomes 90 days after stroke. There were no serious adverse events associated with creatine supplementation. However, it decreased progranulin levels, raising a new possibility of creatine action. This finding needs further exploration to understand the biological significance of creatine-progranulin interaction.
Keywords: creatine; muscle mass; older people; progranulin; sarcopenia; stroke.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- GBD 2015 Mortality and Causes of Death Collaborators Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. Erratum in Lancet 2017, 389, e1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

