Clinical Efficacy of Probiotics for Allergic Rhinitis: Results of an Exploratory Randomized Controlled Trial
- PMID: 39683566
- PMCID: PMC11644003
- DOI: 10.3390/nu16234173
Clinical Efficacy of Probiotics for Allergic Rhinitis: Results of an Exploratory Randomized Controlled Trial
Abstract
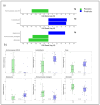
Background: Allergic Rhinitis (AR) is an atopic disease affecting the upper airways of predisposed subjects exposed to aeroallergens. This study evaluates the effects of a mix of specific probiotics (L. acidophilus PBS066, L. rhamnosus LRH020, B. breve BB077, and B. longum subsp. longum BLG240) on symptoms and fecal microbiota modulation in subjects with AR. Methods: Probiotic effects were evaluated at the beginning (T0), at four and eight weeks of treatment (T1 and T2, respectively), and after four weeks of follow-up from the end of treatment (T3) (n = 19) compared to the placebo group (n = 22). AR symptoms and quality of life were evaluated by the mini rhinitis quality of life questionnaire (MiniRQLQ) at each time point. Allergic immune response and fecal microbiota compositions were assessed at T0, T2, and T3. The study was registered on Clinical-Trial.gov (NCT05344352). Results: The probiotic group showed significant improvement in the MiniRQLQ score at T1, T2, and T3 vs. T0 (p < 0.01, p < 0.05, p < 0.01, respectively). At T2, the probiotic group showed an increase in Dorea, which can be negatively associated with allergic diseases, and Fusicatenibacter, an intestinal bacterial genus with anti-inflammatory properties (p-value FDR-corrected = 0.0074 and 0.013, respectively). Conversely, at T3 the placebo group showed an increase in Bacteroides and Ruminococcaceae unassigned, (p-value FDR-corrected = 0.033 and 0.023, respectively) which can be associated with allergies, while the probiotic group showed a significative increase in the Prevotella/Bacteroides ratio (p-value FDR-corrected = 0.023). Conclusions: This probiotic formulation improves symptoms and quality of life in subjects with AR, promoting a shift towards anti-inflammatory and anti-allergic bacterial species in the intestinal microbiota.
Keywords: allergic rhinitis; immune system; inflammation; microbiota; probiotics.
Conflict of interest statement
P. Malfa and D.F. Squarzanti are Synbalance Srl employees. The sponsor did not participate in the study analysis, construction, or decision about article submission.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

