An 8-Week Very Low-Calorie Ketogenic Diet (VLCKD) Alters the Landscape of Obese-Derived Small Extracellular Vesicles (sEVs), Redefining Hepatic Cell Phenotypes
- PMID: 39683581
- PMCID: PMC11643994
- DOI: 10.3390/nu16234189
An 8-Week Very Low-Calorie Ketogenic Diet (VLCKD) Alters the Landscape of Obese-Derived Small Extracellular Vesicles (sEVs), Redefining Hepatic Cell Phenotypes
Abstract
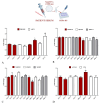
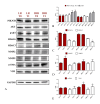
Background. Very low-calorie ketogenic diets (VLCKD) are an effective weight-loss strategy for obese individuals, reducing risks of liver conditions such as non-alcoholic steatohepatitis and fibrosis. Small extracellular vesicles (sEVs) are implicated in liver fibrosis by influencing hepatic cell phenotypes and contributing to liver damage. This study investigates sEVs derived from serum of 60 obese adults categorized into low fibrosis risk (LR) and intermediate/high fibrosis risk (IHR) groups based on FibroScan elastography (FIB E scores, limit value 8 kPa) and all participants underwent an 8-week VLCKD intervention. Methods. The study examines the impact of these sEVs on fibrosis markers, inflammation, and autophagy in a hepatocyte cell line (HEPA-RG) using bioinformatics, RNA sequencing, lipidomics, RT-PCR, and Western blotting before (T0) and after (T1) VLCKD. Results. sEVs from LR patients post-VLCKD reduced fibrosis related gene expression (e.g., ACTA2) and enhanced proteins associated with regeneration and inflammation (e.g., HDAC6). Conversely, sEVs from IHR patients increased fibrosis and inflammation related gene expression (PIK3CB, AKT1, ACTA2) in hepatocytes, raising concerns about VLCKD suitability for IHR patients. IHR sEVs also decreased expression of HDAC10, HDAC6, HDAC3, MMP19, and MMP2, while increasing modulation of p-AKT, α-SMA, and VIM. Conclusion. These findings underscore the critical role of sEVs in regulating inflammation, remodeling, and hepatic stress responses, particularly in IHR patients, and suggest sEVs could complement instrumental evaluations like FibroScan in fibrosis assessment.
Keywords: VLCKD; fibrosis; liver disease; obesity; small extracellular vesicles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Kramer J.R., Lin M., Feng H., Chayanupatkul M., Yu. X., Mapakshi S.R., Desiderio R., Li L., Thrift A.P., El-Serag H.B., et al. Absolute Risk of Hepatocellular Carcinoma in a Large, Geographically and Ethnically Diverse Cohort of Patients with Non-Alcholic Fatty Liver Disease. Gastroenterology. 2017;152:S683. doi: 10.1016/S0016-5085(17)32398-3. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

