Efficacy and Safety of Anthocyanin-Rich Extract in Patients with Ulcerative Colitis: A Randomized Controlled Trial
- PMID: 39683589
- PMCID: PMC11644667
- DOI: 10.3390/nu16234197
Efficacy and Safety of Anthocyanin-Rich Extract in Patients with Ulcerative Colitis: A Randomized Controlled Trial
Abstract
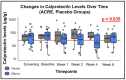
Background: Bilberries are effective in inducing clinical, endoscopic, and biochemical improvement in ulcerative colitis (UC) patients. The aim of this study was to investigate the efficacy of anthocyanin-rich extract (ACRE), the bioactive ingredient of bilberries, in a controlled clinical trial in moderate-to-severe UC. Methods: A multi-center, randomized, placebo-controlled, double-blind study with a parallel group was conducted. Initially, the study was planned for 100 patients; nevertheless, it prematurely ended due to COVID-19. Patients had moderate-to-severe active UC at screening (a Mayo score of 6-12, an endoscopic sub-score ≥ 2) and were randomized at baseline. The primary endpoint was a clinical response (week 8, a total Mayo score reduction ≥ 3 points). Fecal calprotectin (FC) and a centrally read endoscopic response were among the secondary endpoints. Results: Out of 48 patients (6 Swiss centers), 34 were randomized. Eighteen ACRE and eight placebo patients could be analyzed (per protocol set). Half (9/18) of ACRE patients and 3/8 of placebo patients responded clinically (p = 0.278). An improvement in the Mayo score was observed in the ACRE arm (77.8% vs. 62.5% placebo). FC dropped from 1049 ± 1139 to 557 ± 756 μg/g for ACRE but not for the placebo group (947 ± 1039 to 1040 ± 1179; p = 0.035). Serious adverse events were rare. Conclusions: ACRE treatment did not yield significant superiority to the placebo. Furthermore, the placebo response was unusually high. Moreover, there was a significant calprotectin decrease at the end of treatment, indicative of ACRE efficacy in UC.
Keywords: anthocyanin-rich extract (ACRE); bilberries; complementary therapy; inflammatory bowel disease (IBD); ulcerative colitis.
Conflict of interest statement
Luc Biedermann reports fees for consulting/advisory board from Abbvie, MSD, Vifor, Falk, Esocap, Calypso, Ferring, Pfizer, Shire, Takeda, Janssen, and Ewopharma. Michael Doulberis reports traveling fees from Takeda, FALK, Abbvie, as well as consulting fees from Takeda. Philipp Schreiner received consulting fees from Pfizer, Abbvie, Takeda, and Janssen-Cilag and travel support from Falk, UCB, and Pfizer. Benjamin Misselwitz received speaking or traveling fees and/or served on an advisory board with BMS, Abbvie, Takeda, Falk, iQONE, Amgen, MSD, Jansen, Gilead, and Celltrion. BM also received unrestricted research grants from MSD, BMS, and Nestle. Stephan Brand received speaker’s honoraria from Abbvie, Falk Foundation, Ferring, Janssen, Lilly, MSD, Takeda, UCB, and Vifor and participated in advisory boards for Abbvie, BMS, Celgene, Janssen, Lilly, MSD, Pfizer, Pierre Fabre, Roche, Takeda, and UCB. Stephan Brand received an educational grant from Takeda. Hans Herfarth: Consultant Boxer Capital, Celltrion, Fresenius Kabi, ExeGI, Janssen; DSMB member: Galapagos, Finch, Gilead, Ventyx. Research support: Lilly, Novo Nordisk, and Pfizer. Gerhard Rogler declares consulting fees from Abbvie, Augurix, BMS, Boehringer, Calypso, Celgene, FALK, Ferring, Fisher, Genentech, Gilead, Janssen, MSD, Novartis, Pfizer, Phadia, Roche, UCB, Takeda, Tillots, Vifor, Vital Solutions, and Zeller; speaker honoraria from Astra Zeneca, Abbvie, FALK, Janssen, MSD, Pfizer, Phadia, Takeda, Tillots, UCB, Vifor, and Zeller; and grants support from Abbvie, Ardeypharm, Augurix, Calypso, FALK, Flamentera, MSD, Novartis, Pfizer, Roche, Takeda, Tillots, UCB, and Zeller. Author Michael Doulberis was employed by the Gastroklinik. The rest of the authors declare no compelling conflict of interests.
Figures


References
-
- Lang B.M., Ledergerber M., Jordi S.B.U., Krupka N., Biedermann L., Schreiner P., Juillerat P., Wyss J., Vavricka S.R., Zeitz J., et al. Because I’m happy—Positive affect and its predictive value for future disease activity in patients with inflammatory bowel diseases: A retrospective cohort study. Therap Adv. Gastroenterol. 2023;16:1–15. doi: 10.1177/17562848231179335. - DOI - PMC - PubMed
-
- Norouzkhani N., Faramarzi M., Ghodousi Moghadam S., Karimi M.A., Shokri Shirvani J., Bahari A., ShojaeiBaghini M., Eslami S., Tabesh H. Identification of the informational and supportive needs of patients diagnosed with inflammatory bowel disease: A scoping review. Front. Psychol. 2023;14:1055449. doi: 10.3389/fpsyg.2023.1055449. - DOI - PMC - PubMed
-
- Harbord M., Eliakim R., Bettenworth D., Karmiris K., Katsanos K., Kopylov U., Kucharzik T., Molnar T., Raine T., Sebastian S., et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J. Crohns Colitis. 2017;11:769–784. doi: 10.1093/ecco-jcc/jjx009. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

