Butyrate as a Potential Modulator in Gynecological Disease Progression
- PMID: 39683590
- PMCID: PMC11644728
- DOI: 10.3390/nu16234196
Butyrate as a Potential Modulator in Gynecological Disease Progression
Abstract
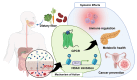
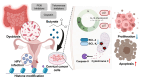
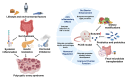
This review investigates the therapeutic potential of butyrate, a short-chain fatty acid (SCFA) produced by gut microbiota, in the prevention and treatment of various gynecological diseases, including polycystic ovary syndrome (PCOS), endometriosis, and gynecologic cancers like cervical and ovarian cancer. These conditions often pose treatment challenges, with conventional therapies offering limited and temporary relief, significant side effects, and a risk of recurrence. Emerging evidence highlights butyrate's unique biological activities, particularly its role as a histone deacetylase (HDAC) inhibitor, which allows it to modulate gene expression, immune responses, and inflammation. In PCOS, butyrate aids in restoring hormonal balance, enhancing insulin sensitivity, and reducing chronic inflammation. For endometriosis, butyrate appears to suppress immune dysregulation and minimize lesion proliferation. Additionally, in cervical and ovarian cancers, butyrate demonstrates anticancer effects through mechanisms such as cell cycle arrest, apoptosis induction, and suppression of tumor progression. Dietary interventions, particularly high-fiber and Mediterranean diets, that increase butyrate production are proposed as complementary approaches, supporting natural microbiota modulation to enhance therapeutic outcomes. However, butyrate's short half-life limits its clinical application, spurring interest in butyrate analogs and probiotics to maintain stable levels and extend its benefits. This review consolidates current findings on butyrate's multifaceted impact across gynecological health, highlighting the potential for microbiota-centered therapies in advancing treatment strategies and improving women's reproductive health.
Keywords: butyrate; cervical cancer; endometrial cancer; endometriosis; gynecological diseases; high-fiber diet; microbiota; ovarian cancer; polycystic ovary syndrome; short-chain fatty acids.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

