Effects of Melissa officinalis Phytosome on Sleep Quality: Results of a Prospective, Double-Blind, Placebo-Controlled, and Cross-Over Study
- PMID: 39683592
- PMCID: PMC11644815
- DOI: 10.3390/nu16234199
Effects of Melissa officinalis Phytosome on Sleep Quality: Results of a Prospective, Double-Blind, Placebo-Controlled, and Cross-Over Study
Abstract
Background: Melissa officinalis standardised extracts, characterised by the presence of hydroxycinnamic acids, have been experimentally demonstrated to be endowed with anti-anxiety and anti-insomnia pharmacological actions. These effects, probably attributable, at least in part, to the role played by rosmarinic acid on GABA-T, have not always been observed in a reproducible manner in humans, perhaps due to the poor bioavailability of these compounds.
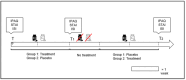
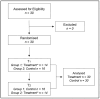
Methods: as nutraceuticals and botanicals could be an alternative option to prescription medications for alleviating symptoms of mild anxiety and insomnia, we have verified in a prospective, double-blind, placebo-controlled, and cross-over study the supporting role on sleep quality played by a Melissa officinalis highly standardised extract, formulated as Phytosome™ (MOP) to improve the oral bioavailability of its active polyphenolic components.
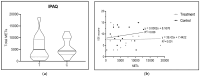
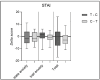
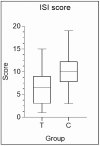
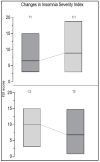
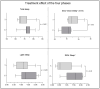
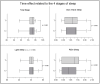
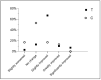
Results: results showed a significant reduction in the ISI score in the treated group, with an average of 6.8 ± 4.1 compared to 9.7 ± 3.7 in the placebo group, indicating a significant reduction of 2.9 points (p = 0.003). The SWS phase duration increased by an average of 15%, while the REM phase decreased by 10%. Additionally, 87% of participants in the treated group reported improved sleep quality, compared to 30% in the placebo group, with significant differences measured by chi-square test (χ2(4) = 21.01, p = 0.0003), highlighting the effects due to Melissa officinalis L. No significant changes in physical activity or anxiety levels were observed.
Conclusions: these findings suggest that MOP may represent a natural and safe alternative to traditional pharmacological treatments for insomnia.
Keywords: GABA-T; hydroxycinnamic acids; oral bioavailability; rosmarinic acid.
Conflict of interest statement
Author F.D.P. is the scientific director of Pharmextracta, Pontenure, Italy; Authors A.B. (Alexander Bertuccioli) and M.C. are Pharmextracta, Pontenure, Italy consultants. The other authors declare no conflicts of interest and no financial relationships.
Figures

References
-
- Zam W., Quispe C., Sharifi-Rad J., López M.D., Schoebitz M., Martorell M., Sharopov F., Fokou P.V.T., Mishra A.P., Chandran D., et al. An Updated Review on The Properties of Melissa officinalis L.: Not Exclusively Anti-Anxiety. Front. Biosci.-Sch. 2022;14:16. doi: 10.31083/j.fbs1402016. - DOI - PubMed
-
- Soltanpour A., Alijaniha F., Naseri M., Kazemnejad A., Heidari M.R. Effects of Melissa officinalis on Anxiety and Sleep Quality in Patients Undergoing Coronary Artery Bypass Surgery: A Double-Blind Randomized Placebo Controlled Trial. Eur. J. Integr. Med. 2019;28:27–32. doi: 10.1016/j.eujim.2019.01.010. - DOI
-
- Cases J., Ibarra A., Feuillère N., Roller M., Sukkar S.G. Pilot Trial of Melissa officinalis L. Leaf Extract in the Treatment of Volunteers Suffering from Mild-to-Moderate Anxiety Disorders and Sleep Disturbances. Mediterr. J. Nutr. Metab. 2010;4:211–218. doi: 10.3233/s12349-010-0045-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

