Peroxiredoxin 4 Ameliorates T-2 Toxin-Induced Growth Retardation in GH3 Cells by Inhibiting Oxidative Stress and Apoptosis
- PMID: 39683652
- PMCID: PMC11643222
- DOI: 10.3390/molecules29235491
Peroxiredoxin 4 Ameliorates T-2 Toxin-Induced Growth Retardation in GH3 Cells by Inhibiting Oxidative Stress and Apoptosis
Abstract
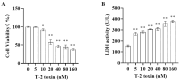
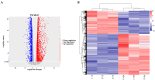
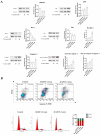
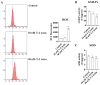
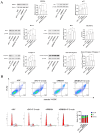
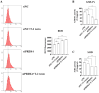
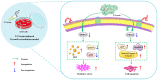
T-2 toxin, a highly toxic type A trichothecene, is a secondary fungal metabolite produced by various Fusarium species. The consumption of food and feed contaminated with T-2 toxin is a major factor contributing to growth retardation, posing significant risks to both human and animal health. However, the specific targets and mechanisms that mitigate T-2 toxin-induced growth retardation remain unclear. In this study, transcriptomic analysis was employed to identify key differentially expressed genes associated with the alleviation of T-2 toxin-induced growth retardation. Peroxiredoxin 4 (PRDX4), a gene linked to oxidative stress and apoptosis, was found to be one of the most downregulated in T-2 toxin-treated GH3 cells, an in vitro model of growth retardation. The experiments demonstrated that T-2 toxin significantly increased reactive oxygen species' production, apoptosis, and cell cycle arrest while reducing the activity of antioxidant enzymes (superoxide dismutase and glutathione peroxidase) and PRDX4 expression in GH3 cells. Furthermore, PRDX4 silencing exacerbated T-2 toxin-induced oxidative stress and apoptosis, whereas PRDX4 overexpression effectively mitigated these effects. These findings highlight the protective role of PRDX4 in counteracting T-2 toxin-induced oxidative stress and apoptosis, suggesting that PRDX4 can serve as a therapeutic target for the treatment of T-2 toxin-induced growth retardation.
Keywords: T-2 toxin; apoptosis; growth retardation; oxidative stress; peroxiredoxin 4.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
The Association of Peroxiredoxin 4 with the Initiation and Progression of Hepatocellular Carcinoma.Antioxid Redox Signal. 2019 Apr 1;30(10):1271-1284. doi: 10.1089/ars.2017.7426. Epub 2018 Jun 11. Antioxid Redox Signal. 2019. PMID: 29687726
-
Protective mechanisms involving enhanced mitochondrial functions and mitophagy against T-2 toxin-induced toxicities in GH3 cells.Toxicol Lett. 2018 Oct 1;295:41-53. doi: 10.1016/j.toxlet.2018.05.041. Epub 2018 Jun 2. Toxicol Lett. 2018. PMID: 29870751
-
T-2 toxin exposure induces apoptosis in rat ovarian granulosa cells through oxidative stress.Environ Toxicol Pharmacol. 2013 Sep;36(2):493-500. doi: 10.1016/j.etap.2013.03.017. Epub 2013 Apr 11. Environ Toxicol Pharmacol. 2013. PMID: 23811107
-
Oxidative stress-mediated cytotoxicity and metabolism of T-2 toxin and deoxynivalenol in animals and humans: an update.Arch Toxicol. 2014 Jul;88(7):1309-26. doi: 10.1007/s00204-014-1280-0. Epub 2014 Jun 4. Arch Toxicol. 2014. PMID: 24894432 Review.
-
Research progress on the role and molecular mechanism of PRDX4 in malignant tumors.Bull Cancer. 2025 May;112(5):519-526. doi: 10.1016/j.bulcan.2025.02.009. Epub 2025 Mar 28. Bull Cancer. 2025. PMID: 40157831 Review.
Cited by
-
Dual Signal Enhancement by Magnetic Separation and Split Aptamer for Ultrasensitive T-2 Toxin Detection.Molecules. 2025 Jul 4;30(13):2853. doi: 10.3390/molecules30132853. Molecules. 2025. PMID: 40649367 Free PMC article.
References
-
- Wu Q., Qin Z., Kuca K., You L., Zhao Y., Liu A., Musilek K., Chrienova Z., Nepovimova E., Oleksak P., et al. An update on t-2 toxin and its modified forms: Metabolism, immunotoxicity mechanism, and human exposure assessment. Arch. Toxicol. 2020;94:3645–3669. doi: 10.1007/s00204-020-02899-9. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

