Emerging Fluorescent Nanoparticles for Non-Invasive Bioimaging
- PMID: 39683753
- PMCID: PMC11643714
- DOI: 10.3390/molecules29235594
Emerging Fluorescent Nanoparticles for Non-Invasive Bioimaging
Abstract
Fluorescence-based techniques have great potential in the field of bioimaging and could bring tremendous progress in microbiology and biomedicine. The most essential element in these techniques is fluorescent nanomaterials. The use of fluorescent nanoparticles as contrast agents for bioimaging is a large topic to cover. The purpose of this mini-review is to give the reader an overview of biocompatible and biodegradable fluorescent nanoparticles that are emerging nanomaterials for use in fluorescent bioimaging. In addition to the biocompatibility of these nanomaterials, biodegradability is considered a necessity for short-term sustainable bioimaging. Firstly, the main requirements for bioimaging are raised, and a few existing fluorescent nanoprobes are discussed. Secondly, a few inert biocompatible fluorescent nanomaterials for long-term bioimaging that have been, to some extent, demonstrated as fluorescent probes are reviewed. Finally, a few biocompatible and biodegradable nanomaterials for short-term bioimaging that are evolving for bioimaging applications are discussed. Together, these advancements signal a transformative leap toward sustainability and functionality in biomedical imaging.
Keywords: biocompatibility; biodegradability; bioimaging; fluorescence; nanoparticles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



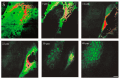
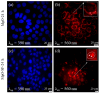
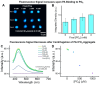
References
-
- Gao Z., Hao Y., Zheng M., Chen Y. A fluorescent dye with large Stokes shift and high stability: Synthesis and application to live cell imaging. RSC Adv. 2017;7:7604–7609. doi: 10.1039/C6RA27547H. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

