Irisin Attenuates Neuroinflammation Targeting the NLRP3 Inflammasome
- PMID: 39683782
- PMCID: PMC11643767
- DOI: 10.3390/molecules29235623
Irisin Attenuates Neuroinflammation Targeting the NLRP3 Inflammasome
Abstract
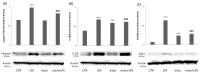
Neuroinflammation is defined as an immune response involving various cell types, particularly microglia, which monitor the neuroimmune axis. Microglia activate in two distinct ways: M1, which is pro-inflammatory and capable of inducing phagocytosis and releasing pro-inflammatory factors, and M2, which has anti-inflammatory properties. Inflammasomes are large protein complexes that form in response to internal danger signals, activating caspase-1 and leading to the release of pro-inflammatory cytokines such as interleukin 1β. Irisin, a peptide primarily released by muscles during exercise, was examined for its effects on BV2 microglial cells in vitro. Even at low concentrations, irisin was observed to influence the NLRP3 inflammasome, showing potential as a neuroprotective and anti-inflammatory agent after stimulation with lipopolysaccharides (LPSs). Irisin helped maintain microglia in their typical physiological state and reduced their migratory capacity. Irisin also increased Arg-1 protein expression, a marker of M2 polarization, while downregulating NLRP3, Pycard, caspase-1, IL-1β, and CD14. The results of this study indicate that irisin may serve as a crucial mediator of neuroprotection, thus representing an innovative tool for the prevention of neurodegenerative diseases.
Keywords: inflammasome; irisin; microglia; neuroinflammation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Bliederhaeuser C., Grozdanov V., Speidel A., Zondler L., Ruf W.P., Bayer H., Kiechle M., Feiler M.S., Freischmidt A., Brenner D., et al. Age-dependent defects of alpha-synuclein oligomer uptake in microglia and monocytes. Acta Neuropathol. 2016;131:379–391. doi: 10.1007/s00401-015-1504-2. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

