Graphene Oxide Nanoparticles for Photothermal Treatment of Hepatocellular Carcinoma Using Low-Intensity Femtosecond Laser Irradiation
- PMID: 39683809
- PMCID: PMC11643931
- DOI: 10.3390/molecules29235650
Graphene Oxide Nanoparticles for Photothermal Treatment of Hepatocellular Carcinoma Using Low-Intensity Femtosecond Laser Irradiation
Abstract
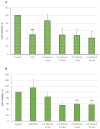
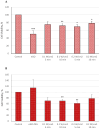
Graphene oxide-mediated photothermal therapy using femtosecond lasers has recently shown promise in treating hepatocellular carcinoma. However, significant work remains to optimize irradiation parameters for specific nanoparticle types and cancer cells to improve nanomaterial-mediated photothermal anticancer therapy. This study investigated the photothermal potential of nGO and nGO-PEG nanoparticles (NPs) combined with femtosecond laser irradiation at 515 nm and 1030 nm wavelengths, with varying power (0.1 and 0.2 W/cm2) and duration (5 and 10 min), to optimize photothermal therapy for hepatocellular carcinoma. Conversion efficiency of NPs, morphology and viability of HepG2 and normal MDCK cells after treatments were evaluated using an electronic thermometer, phase-contrast microscopy, and WST-1 assay. The results revealed that nGO-PEG NPs exhibited better photothermal efficiency than nGO, with 515 nm of irradiation inducing a temperature increase up to 19.1 °C compared to 4.7 °C with 1030 nm of light. Laser exposure to 515 nm significantly reduced HepG2 cell viability, with the most intense conditions (10 min at 0.2 W/cm2) causing a decrease of up to 58.2% with nGO and 43.51% with nGO-PEG. Normal MDCK cells showed minimal impact or a slight viability increase, especially with nGO-PEG. Combined treatment with laser irradiation and NPs induced significant morphological changes in HepG2 cells, including cell detachment and apoptotic-like characteristics, particularly with 1030 nm of irradiation. MDCK cells exhibited minimal morphological changes, with some recovery observed under lower energy conditions. These findings suggest that low-energy lasers and engineered nanomaterials could provide a minimally invasive approach to photothermal cancer therapy with reduced side effects.
Keywords: 1030 nm light; 515 nm light; HepG2 cells; MDCK cells; cell viability; pulse laser.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Asafo-Agyei K.O., Samant H. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2022. Hepatocellular Carcinoma. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

