Bioguided Identification of Polymethoxyflavones as Novel Vascular CaV1.2 Channel Blockers from Citrus Peel
- PMID: 39683852
- PMCID: PMC11642981
- DOI: 10.3390/molecules29235693
Bioguided Identification of Polymethoxyflavones as Novel Vascular CaV1.2 Channel Blockers from Citrus Peel
Abstract
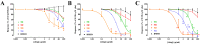
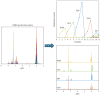
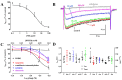
The huge amount of citrus peel produced worldwide represents an economic burden for society. However, this agricultural by-product is a rich source of natural molecules, potentially endowed with interesting pharmacological activities. In this regard, we decided to investigate if the polymethoxyflavones contained in citrus peel waste could be exploited as novel vasorelaxant agents. A hydroalcoholic blond orange (Citrus sinensis) peel extract, obtained by ultrasonication, was partitioned in dichloromethane. Column chromatography allowed for the isolation of four polymethoxyflavones, namely, scutellarein tetramethyl ether, nobiletin, tangeretin, and sinensetin, identified by nuclear magnetic resonance (NMR) spectroscopy and UPLC-HRMS/MS and confirmed by multivariate curve resolution of NMR fractional spectra. The four molecules showed interesting in vitro vasorelaxant activity, at least, in part, due to the blockade of smooth muscle CaV1.2 channels. Molecular modeling and docking analysis elucidated the binding mode of the polymethoxyflavones at the homology model of the rat CaV1.2c subunit and provided the structural basis to rationalise the highest activity of scutellarein tetramethyl ether in the set and the dramatic effect of the additional methoxy group occurring in nobiletin and sinensetin. In conclusion, citrus peel can be considered a freely available, valuable source of vasoactive compounds worthy of pharmaceutical and/or nutraceutical exploitation.
Keywords: CaV1.2 channel blockers; docking studies; multivariate curve resolution; polymethoxyflavones.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Yi L., Ma S., Ren D. Phytochemistry and Bioactivity of Citrus Flavonoids: A Focus on Antioxidant, Anti-Inflammatory, Anticancer and Cardiovascular Protection Activities. Phytochem. Rev. 2017;16:479–511. doi: 10.1007/s11101-017-9497-1. - DOI
-
- Owis A.I. Citrus Polymethoxyflavones: Biofunctional Molecules of Therapeutic Interest. Stud. Nat. Prod. Chem. 2018;59:509–530.
-
- Yuan S., Gao P., Wu S., Liang X., Xiao Y., Tu P., Jiang Y. Rapid and Comprehensive Metabolites Identification of 5-Demethylnobiletin in Rats Using UPLC/Triple-TOF-MS/MS Based on Multiple Mass Defect Filter and Their Neuroprotection against Ferroptosis. J. Pharm. Biomed. Anal. 2024;238:115842. doi: 10.1016/j.jpba.2023.115842. - DOI - PubMed
-
- Du K., Zheng C., Kuang Z., Sun Y., Wang Y., Li S., Meng D. Gastroprotective Effect of Eupatilin, a Polymethoxyflavone from Artemisia Argyi H.Lév. & Vaniot, in Ethanol-Induced Gastric Mucosal Injury via NF-ΚB Signaling Pathway. J. Ethnopharmacol. 2024;318:116986. doi: 10.1016/j.jep.2023.116986. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

