Recent Progress on the Synthesis, Morphological Topography, and Battery Applications of Polypyrrole-Based Nanocomposites
- PMID: 39684021
- PMCID: PMC11644454
- DOI: 10.3390/polym16233277
Recent Progress on the Synthesis, Morphological Topography, and Battery Applications of Polypyrrole-Based Nanocomposites
Abstract
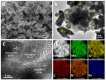
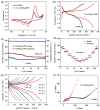
Polypyrrole (PPy)-based nanocomposite materials are of great interest to the scientific community owing to their usefulness in designing state-of-the-art industrial applications, such as fuel cells, catalysts and sensors, energy devices, and especially batteries. However, the commercialization of these materials has not yet reached a satisfactory level of implementation. More research is required for the design and synthesis of PPy-based composite materials for numerous types of battery applications. Due to the rising demand for environmentally friendly, cost-effective, and sustainable energy, battery applications are a significant solution to the energy crisis, utilizing suitable materials like PPy-based composites. Among the conducting polymers, PPy is considered an important class of materials owing to their ease of synthesis, low cost, environmentally friendly nature, and so on. In this context, PPy-based nanocomposites may be very promising due to their nanostructural properties and distinct morphological topography, which are vital concerns for their applications for battery applications. Such features of PPy-based nanocomposites make them particularly promising for next-generation electrode materials. However, the design and fabrication of appropriate PPy-based nanocomposites for battery applications is still a challenging area of research. This review paper describes the current progress on the synthesizing of PPy-based composites for battery applications along with their morphological topography. We discussed here the recent progress on the synthesis of different PPy-based composites, including PPy/S, PPy/MnOx, MWCNT/PPy, V2O5/PPy, Cl-doped PPy/rGO, and Fe/α-MnO2@PPy composites, by a polymerization approach for numerous battery applications. The insights presented in this review aim to provide a comprehensive reference for the future development of PPy-based composites in battery technology.
Keywords: and battery applications; conductive polymers; electrode material; morphology; nanocomposites; polymerization approach; polypyrrole; synthesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Yuvika S., Varsha S.P., Harish M., Anil K. A review on synthetic strategies and gas sensing approach for polypyrrole-based hybrid nanocomposites. Polym. Eng. Sci. 2021;61:2949–2973.
-
- Bhadra S., Khastgir D., Singha N.K., Lee J.H. Progress in preparation, processing and applications of polyaniline. Prog. Polym. Sci. 2009;34:783–810. doi: 10.1016/j.progpolymsci.2009.04.003. - DOI
-
- Chandramika B., Swapan K.D. Interfacial synthesis of polypyrrole/graphene composites and investigation of their optical, electrical and electrochemical properties. Polym. Int. 2014;63:1439–1446.
-
- Abdirahman Y., Mohammad A.-S., Salah A.-E., Gils A. Synthesis and Characterization of Conductive Polypyrrole: The Influence of the Oxidants and Monomer on the Electrical, Thermal, and Morphological Properties. Int. J. Polym. Sci. 2018;2018:4191747.
-
- Ramesan M.T., Santhi V. In situ synthesis, characterization, conductivity studies of polypyrrole/silver doped zinc oxide nanocomposites and their application for ammonia gas sensing. J. Mater. Sci. Mater. Electron. 2017;28:18804–18814. doi: 10.1007/s10854-017-7830-5. - DOI
Publication types
LinkOut - more resources
Full Text Sources

