Silver-Catalyzed Aqueous Electrochemical Valorization of Soda Lignin into Aliphatics and Phenolics
- PMID: 39684070
- PMCID: PMC11644402
- DOI: 10.3390/polym16233325
Silver-Catalyzed Aqueous Electrochemical Valorization of Soda Lignin into Aliphatics and Phenolics
Abstract
Transitioning from crude oil to renewable sources of carbon-based chemicals is critical for advancing sustainable development. Lignin, a wood-derived biomacromolecule, holds great potential as a renewable feedstock, but efficient depolymerization and dearomatization methods are required to fully unlock its potential. In this investigation, we present a silver-catalyzed aqueous electrocatalytic method for the selective depolymerization and partial dearomatization of soda lignin under mild, ambient conditions. Utilizing a water/sodium carbonate solvent system and a silver electrode to mediate the electrochemical reduction, we achieved significant lignin depolymerization over reaction times ranging from 5 to 20 h. Analysis by nuclear magnetic resonance (NMR) and high-resolution mass spectrometry (HRMS) revealed sodium levulinate, sodium acetate, and sodium formate as the main aliphatic products, alongside various aromatic species in the depolymerized lignin products (DL). This selective conversion of lignin into both valuable aromatic compounds and reactive aliphatic intermediates offers promising opportunities for further synthesis of a wide range of organic chemicals, contributing to the development of a more sustainable and circular economy.
Keywords: dearomatization; depolymerization; electrocatalysis; lignin; silver.
Conflict of interest statement
Author Marcella Frauscher was employed by AC2T Research GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare the study received fundings from COMET InTribology project (FFG No. 872176,347 project coordinator: AC2T research GmbH). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it.
Figures

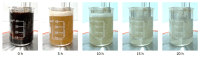
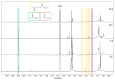


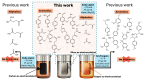
References
-
- Zhou N., Thilakarathna W.P.D.W., He Q.S., Rupasinghe H.P.V. A Review: Depolymerization of Lignin to Generate High-Value Bio-Products: Opportunities, Challenges, and Prospects. Front. Energy Res. 2022;9:758744. doi: 10.3389/fenrg.2021.758744. - DOI
-
- Luo J., Liu T.L. Electrochemical Valorization of Lignin: Status, Challenges, and Prospects. J. Bioresour. Bioprod. 2023;8:1–14. doi: 10.1016/j.jobab.2022.11.003. - DOI
-
- Lindenbeck L.M., Barra V.C., Dahlhaus S., Brand S., Wende L.M., Beele B.B., Schebb N.H., Rodrigues B.V.M., Slabon A. Organic Chemicals from Wood: Selective Depolymerization and Dearomatization of Lignin via Aqueous Electrocatalysis. ChemSusChem. 2024;17:e202301617. doi: 10.1002/cssc.202301617. - DOI - PubMed
LinkOut - more resources
Full Text Sources

