Investigating the Relationship Between the Emulsification Parameters and Physical-Chemical Properties of Poly(D,L-lactic acid) Particles for Dermal Fillers
- PMID: 39684140
- PMCID: PMC11644387
- DOI: 10.3390/polym16233395
Investigating the Relationship Between the Emulsification Parameters and Physical-Chemical Properties of Poly(D,L-lactic acid) Particles for Dermal Fillers
Abstract
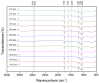
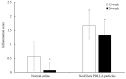
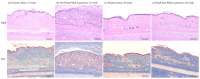
Poly(L-lactic acid) (PLLA) and poly(D,L-lactic acid) (PDLLA) particles have been applied as dermal fillers for soft-tissue augmentation because they can induce foreign-body reactions, resulting in fibroblast proliferation and collagen formation. Although PLLA and PDLLA fillers are safe and biocompatible, clinical complications such as nodules and granulomas have been reported, possibly due to incomplete reconstitution. PDLLA particles were prepared via emulsification in this study, and three stirring speeds were investigated when adding PDLLA into carboxymethyl cellulose solution. The particle size, molecular weight of PDLLA, optical rotation, pH value, osmotic pressure, and reconstitution time were analyzed. A rabbit dorsal ear model was established to evaluate the soft-tissue augmentation of a commercial PDLLA filler. The results demonstrated that the stirring speed affected the particle size, but not other physical-chemical properties of the PDLLA particles. All the PDLLA particles were reconstituted in less than 7 min, which is faster than the process for the other commercial PDLLA dermal filler products. In addition, the PDLLA particles could induce inflammation and fibroblast proliferation. Although the PDLLA particles generated in this study have not yet been investigated in vivo, the results demonstrated here suggest their potential for application as dermal fillers.
Keywords: carboxymethyl cellulose; dermal filler; emulsification; poly(D,L-lactic acid) acid particle; reconstitution; soft-tissue augmentation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Sedush N.G., Kalinin K.T., Azarkevich P.N., Gorskaya A.A. Physicochemical characteristics and hydrolytic degradation of polylactic acid dermal fillers: A comparative study. Cosmetics. 2023;10:110. doi: 10.3390/cosmetics10040110. - DOI
-
- Benedetto A.V., Lewis A.T. Injecting 1000 centistoke liquid silicone with ease and precision. Dermatol. Surg. 2003;29:211–214. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

