Antimicrobial and Antifungal Action of Biogenic Silver Nanoparticles in Combination with Antibiotics and Fungicides Against Opportunistic Bacteria and Yeast
- PMID: 39684204
- PMCID: PMC11641717
- DOI: 10.3390/ijms252312494
Antimicrobial and Antifungal Action of Biogenic Silver Nanoparticles in Combination with Antibiotics and Fungicides Against Opportunistic Bacteria and Yeast
Abstract
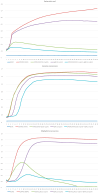
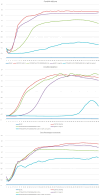
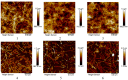
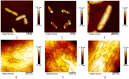
The development of multidrug resistance by pathogenic bacteria and yeast is a significant medical problem that needs to be addressed. One possible answer could be the combined use of antibiotics and silver nanoparticles, which have different mechanisms of antimicrobial action. In the same way, these nanoparticles can be combined with antifungal agents. Biogenic silver nanoparticles synthesized using environmentally friendly biosynthesis technology using extracts of biologically active plants are an effective nanomaterial that needs to be comprehensively investigated for implementation into medical practice. In this study, the synergistic effects arising from their combined use with antibiotics and fungicides against various bacteria and yeasts were studied. The following methods were used: disco-diffusion analysis and construction of plankton culture growth curves. The synergistic effect of silver nanoparticles and antibiotics (fungicides) has been determined. Effective concentrations of substances were established, recommendations for the studied pathogenic species were presented, and the effect of destruction of the bacterial membrane was illustrated. The most significant synergistic effect was manifested in pathogenic candida and brewer's yeast.
Keywords: Alcanivorax borkumensis; Candida; Pseudomonas putida; antibiotic; antifungal action; antimicrobial action; biogenic silver nanoparticles; fungicide.
Conflict of interest statement
The authors declare no conflicts of interest. All authors have read and agreed to the published version of the manuscript.
Figures

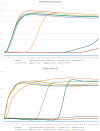
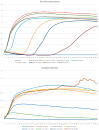

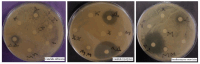
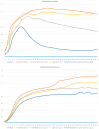
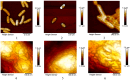

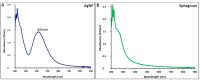
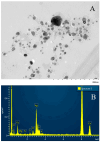

Similar articles
-
Synthesis, characterization and evaluation of antimicrobial and cytotoxic activities of biogenic silver nanoparticles synthesized from Streptomyces xinghaiensis OF1 strain.World J Microbiol Biotechnol. 2018 Jan 5;34(2):23. doi: 10.1007/s11274-017-2406-3. World J Microbiol Biotechnol. 2018. PMID: 29305718 Free PMC article.
-
A review on biosynthesis of silver nanoparticles and their biocidal properties.J Nanobiotechnology. 2018 Feb 16;16(1):14. doi: 10.1186/s12951-018-0334-5. J Nanobiotechnology. 2018. PMID: 29452593 Free PMC article. Review.
-
Combined efficacy of biologically synthesized silver nanoparticles and different antibiotics against multidrug-resistant bacteria.Int J Nanomedicine. 2013;8:3187-95. doi: 10.2147/IJN.S49284. Epub 2013 Aug 20. Int J Nanomedicine. 2013. PMID: 23986635 Free PMC article.
-
Silver nanoparticles: the powerful nanoweapon against multidrug-resistant bacteria.J Appl Microbiol. 2012 May;112(5):841-52. doi: 10.1111/j.1365-2672.2012.05253.x. Epub 2012 Mar 28. J Appl Microbiol. 2012. PMID: 22324439 Review.
-
Silver nanoparticles from Pilimelia columellifera subsp. pallida SL19 strain demonstrated antifungal activity against fungi causing superficial mycoses.J Basic Microbiol. 2017 Sep;57(9):793-800. doi: 10.1002/jobm.201700121. Epub 2017 Jul 3. J Basic Microbiol. 2017. PMID: 28670763
Cited by
-
Mycogenic Silver Nanoparticles: Promising Antimicrobials with Fungistatic Properties.Int J Mol Sci. 2025 Jul 10;26(14):6639. doi: 10.3390/ijms26146639. Int J Mol Sci. 2025. PMID: 40724889 Free PMC article.
References
-
- de Oliveira Santos J.V., da Costa Júnior S.D., de Fátima Ramos dos Santos Medeiros S.M., Cavalcanti I.D.L., de Souza J.B., Coriolano D.L., da Silva W.R.C., Menezes M.H., Alves E., Cavalcanti I.M.F. Panorama of bacterial infections caused by epidemic resistant strains. Curr. Microbiol. 2022;79:175. doi: 10.1007/s00284-022-02875-9. - DOI - PMC - PubMed
-
- Fernández-Martínez N.F., Rivera-Izquierdo M., Ortiz-González-Serna R., Martínez-Ruiz V., Lardelli-Claret P., Aginagalde-Llorente A.H., del Carmen Valero-Ubierna M., Vergara-Díaz M.A., Lorusso N. Healthcare-associated infections by multidrug-resistant bacteria in Andalusia, Spain, 2014 to 2021. Eurosurveillance. 2023;28:2200805. doi: 10.2807/1560-7917.ES.2023.28.39.2200805. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

