Endothelial Dysfunction and Impaired Wound Healing Following Radiation Combined Skin Wound Injury
- PMID: 39684207
- PMCID: PMC11641134
- DOI: 10.3390/ijms252312498
Endothelial Dysfunction and Impaired Wound Healing Following Radiation Combined Skin Wound Injury
Abstract
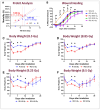
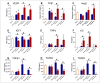
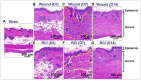
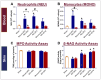
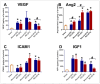
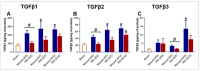
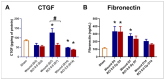
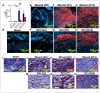
Currently, there are no U.S. Food and Drug Administration (FDA)-approved medical countermeasures (MCMs) for radiation combined injury (RCI), partially due to limited understanding of its mechanisms. Our previous research suggests that endothelial dysfunction may contribute to a poor prognosis of RCI. In this study, we demonstrated an increased risk of mortality, body weight loss, and delayed skin wound healing in RCI mice compared to mice with skin wounds alone or radiation injury (RI) 30 days post-insult. Furthermore, we evaluated biomarkers of endothelial dysfunction, inflammation, and impaired wound healing in mice at early time points after RCI. Mice were exposed to 9.0 Gy total-body irradiation (TBI) followed by skin wound. Samples were collected on days 3, 7, and 14 post-TBI. Endothelial dysfunction markers were measured by ELISA, and skin wound healing was assessed histologically. Our results show that endothelial damage and inflammation are more severe and persistent in the RCI compared to the wound-alone group. Additionally, RCI impairs granulation tissue formation, reduces myofibroblast presence, and delays collagen deposition, correlating with more severe endothelial damage. TGF signaling may play a key role in this impaired healing. These findings suggest that targeting the endothelial dysfunction and TGF-β pathways may provide potential therapeutic strategies for improving delayed wound healing in RCI, which could subsequently influence outcomes such as survival after RCI.
Keywords: TGFβ expression; collagen deposition; endothelial dysfunction; granulation tissue formation; impaired wound healing; myofibroblast; radiation combined skin wound injury; systemic and local proinflammatory response.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The opinions and assertions expressed herein are those of the author(s) and do not reflect the official policy or position of the Uniformed Services University of the Health Sciences or the Department of Defense.
Figures
References
-
- Wang L., Zhai M., Lin B., Cui W., Hull L., Li X., Anderson M.N., Smith J.T., Umali M.V., Jiang S., et al. PEG-G-CSF and L-Citrulline Combination Therapy for Mitigating Skin Wound Combined Radiation Injury in a Mouse Model. Radiat. Res. 2021;196:113–127. doi: 10.1667/RADE-20-00151.1. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

