Microbiome-Derived Trimethylamine N-Oxide (TMAO) as a Multifaceted Biomarker in Cardiovascular Disease: Challenges and Opportunities
- PMID: 39684223
- PMCID: PMC11641139
- DOI: 10.3390/ijms252312511
Microbiome-Derived Trimethylamine N-Oxide (TMAO) as a Multifaceted Biomarker in Cardiovascular Disease: Challenges and Opportunities
Abstract
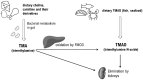
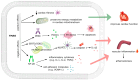
Biomarkers play a crucial role in various stages of disease management, including screening, diagnosis, prediction, prognosis, treatment, and safety monitoring. Although they are powerful tools in disease diagnosis, management, and drug development, identifying and validating reliable biomarkers remains a significant challenge. Among potential microbiome-derived biomarkers, trimethylamine N-oxide (TMAO) has gained notable attention for its link to atherosclerosis and cardiovascular risk. However, despite the growing body of research on TMAO, its practical application in clinical settings for disease management and patient outcome enhancement is still not a reality. This paper presents recent data on the utility of TMAO as a cardiovascular biomarker, categorized by its various roles: diagnostic, prognostic, susceptibility/risk, monitoring, pharmacodynamic/response, predictive, and safety. It also briefly discusses research on TMAO's potential role in cardiovascular disease development. While TMAO shows promise, particularly in prognostic applications, its reliability as a biomarker has been inconsistent across studies. These variances may result from several confounding factors that affect TMAO plasma levels, including diet, kidney function, and demographic variables. The review aims to elucidate the specific contexts in which TMAO can be valuable, potentially leading to more personalized and effective management of cardiovascular disease.
Keywords: bacterial metabolites; biomarker; cardiovascular marker; surrogate marker; trimethylamine oxide.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- FDA-NIH Biomarker Working Group . BEST (Biomarkers, EndpointS, and Other Tools) Resource. Food and Drug Administration (US); Silver Spring, MD, USA: 2016. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

