Impact of Missense Mutations on AFB1 Metabolism in Bovine Cytochrome P4503A Isoforms: A Computational Mutagenesis and Molecular Docking Analysis
- PMID: 39684241
- PMCID: PMC11640871
- DOI: 10.3390/ijms252312529
Impact of Missense Mutations on AFB1 Metabolism in Bovine Cytochrome P4503A Isoforms: A Computational Mutagenesis and Molecular Docking Analysis
Abstract
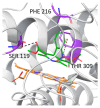
Cytochrome P450 3A (CYP3A) enzymes catalyze the metabolism of a wide range of endogenous and exogenous compounds. Genetic variations in the 3 CYP3A isoforms (CYP3A28, CYP3A74, and CYP3A76) may influence their expression and activity, leading to inter-individual differences in xenobiotic metabolism. In domestic cattle, understanding how genetic variations modulate CYP3A activity is crucial for both its therapeutic implications (clinical efficacy and adverse drug effects) and food safety (residues in foodstuff). Here, we updated the variant calling of CYP3As in 300 previously sequenced Piedmontese beef cattle, using the most recent reference genome, which contains an updated, longer sequence for CYP3A28. All but one previously identified missense variants were confirmed and a new variant, R105W in CYP3A28, was discovered. Through computational mutagenesis and molecular docking, we computationally predicted the impact of all identified CYP3A variant enzymes on protein stability and their affinity for aflatoxin B1 (AFB1), a potent carcinogen and food contaminant. For CYP3A28, we also computationally predicted its affinity for the probe substrate nifedipine (NIF). We found that CYP3A28 with R105W variant cannot accommodate NIF nor AFB1 in the binding pocket, thus affecting their metabolism. Our work provides computational foundation and prioritized ranking of CYP3A variants for future experimental validations.
Keywords: CYP3A; Piedmontese cattle breed; aflatoxin B1; cattle genetic variants; computational mutagenesis; cytochrome P450; molecular docking; nifedipine; single nucleotide variants.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Ohtsuki S., Schaefer O., Kawakami H., Inoue T., Liehner S., Saito A., Ishiguro N., Kishimoto W., Ludwig-Schwellinger E., Ebner T., et al. Simultaneous Absolute Protein Quantification of Transporters, Cytochromes P450, and UDP-Glucuronosyltransferases as a Novel Approach for the Characterization of Individual Human Liver: Comparison with mRNA Levels and Activities. Drug Metab. Dispos. 2012;40:83–92. doi: 10.1124/dmd.111.042259. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

