The Role of lncRNAs in the Protective Action of Tamoxifen on the Ovaries of Tumor-Bearing Rats Receiving Cyclophosphamide
- PMID: 39684249
- PMCID: PMC11640806
- DOI: 10.3390/ijms252312538
The Role of lncRNAs in the Protective Action of Tamoxifen on the Ovaries of Tumor-Bearing Rats Receiving Cyclophosphamide
Abstract
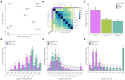
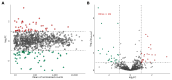
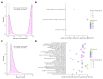
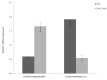
Infertility due to ovarian toxicity is a common side effect of cancer treatment in premenopausal women. Tamoxifen (TAM) is a selective estrogen receptor modulator that prevented radiation- and chemotherapy-induced ovarian failure in preclinical studies. In the current study, we examined the potential regulatory role of long noncoding RNAs (lncRNAs) in the mechanism of action of TAM in the ovaries of tumor-bearing rats receiving cyclophosphamide (CPA) as cancer therapy. We identified 166 lncRNAs, among which 49 were demonstrated to be differentially expressed (DELs) in the ovaries of rats receiving TAM and CPA compared to those receiving only CPA. A total of 24 DELs were upregulated and 25 downregulated by tamoxifen. The identified DELs shared the characteristics of noncoding RNAs described in other reproductive tissues. Eleven of the identified DELs displayed divergent modes of action, regulating target transcripts via both cis- and trans-acting pathways. Functional enrichment analysis revealed that, among target genes ascribed to the identified DELs, the majority were involved in apoptosis, cell adhesion, immune response, and ovarian aging. The presented data suggest that the molecular mechanisms behind tamoxifen's protective effects in the ovaries may involve lncRNA-dependent regulation of critical signaling pathways related to inhibition of follicular transition and ovarian aging, along with the suppression of apoptosis and regulation of cell adhesion. Employing a tumor-bearing animal model undergoing chemotherapy, which accurately reflects the conditions of mammary cancer, reinforces the obtained results. Given that tamoxifen remains a key player in the management and prevention of breast cancer, understanding its ovarian-specific actions in cancer patients is crucial and requires detailed functional studies to clarify the underlying molecular mechanisms.
Keywords: cancer treatment; cyclophosphamide; fertility preservation; long noncoding RNA (lncRNA); ovarian reserve; tamoxifen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Ugai T., Sasamoto N., Lee H.-Y., Ando M., Song M., Tamimi R.M., Kawachi I., Campbell P.T., Giovannucci E.L., Weiderpass E., et al. Is Early-Onset Cancer an Emerging Global Epidemic? Current Evidence and Future Implications. Nat. Rev. Clin. Oncol. 2022;19:656–673. doi: 10.1038/s41571-022-00672-8. - DOI - PMC - PubMed
-
- Zhao J., Xu L., Sun J., Song M., Wang L., Yuan S., Zhu Y., Wan Z., Larsson S., Tsilidis K., et al. Global Trends in Incidence, Death, Burden and Risk Factors of Early-Onset Cancer from 1990 to 2019. BMJ Oncol. 2023;2:e000049. doi: 10.1136/bmjonc-2023-000049. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

