Neutrophils in the Spotlight-An Analysis of Neutrophil Function and Phenotype in ARDS
- PMID: 39684262
- PMCID: PMC11641705
- DOI: 10.3390/ijms252312547
Neutrophils in the Spotlight-An Analysis of Neutrophil Function and Phenotype in ARDS
Abstract
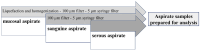
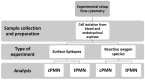
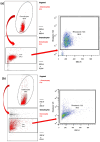
Acute respiratory distress syndrome (ARDS) is a complex disease pattern in which pathogenesis polymorphonuclear neutrophil granulocytes (PMN) play a key role. In previous experiments, we could show that interaction with collagen III (an important component of pulmonary tissue) is a possible trigger of neutrophil reactive oxygen species (ROS) production. To investigate possible correlations, further elucidate ARDS pathophysiology, and maybe find pharmacological targets, we evaluated PMNs from blood (circulating PMNs: cPMNs) and tracheal secretion (tPMNs) from patients with and without ARDS with regard to function and phenotype. Blood samples and tracheal secretions were obtained from intensive care patients with and without ARDS. Isolation of cPMN was performed by density-gradient gravity sedimentation without centrifugation. For tPMN isolation, endotracheal aspirate was filtered, and tPMNs were separated from the remaining aspirate using a particle filter. Specific surface epitopes (CD66b, CD62L, fMLP-receptor, LOX-1, CD49d, CD29, CD11b) of the isolated PMN cells were labeled with antibody-coupled dyes and analyzed by flow cytometry. Neutrophil ROS production before and after activation with N-formyl-methyl-leucyl-phenylalanine (fMLP) and tumor necrosis factor α (TNFα) was quantified using rhodamine-123. In addition, a qualitative cytological hematoxylin-eosin (HE) staining was performed with a portion of the secretion. tPMNs were observed in both bloody and mucosal tracheal secretions from ARDS patients. The epitope distribution on cPMNs and tPMNs differed significantly in patients with and without ARDS: tPMNs generally showed increased expression of CD66b, LOX-1 and fMLP-receptor compared to cPMNs, and decreased expression of CD62L. The CD49d levels of all cPMNs were at the same level as tPMNs in ARDS, whereas CD49d expression was increased on tPMNs without ARDS. ROS production was significantly stimulated by fMLP/TNFα in cPMNs regardless of the patient group, while it was similarly increased in tPMNs with and without stimulation. Increased expression of CD66b, LOX-1 and fMLP-receptor on tPMNs indicated a higher activity status compared to cPMNs. Increased CD49d expression on tPMNs without ARDS marks different PMN surface changes in lung disease. PMNs appear to be in a more activated state in lung secretions than in blood, as indicated by higher CD66b and lower CD62L expression, higher constitutive ROS production and lower excitability with fMLP and TNFα. In the context of possible CD49d-triggered ROS production, it is noteworthy that CD49d is downregulated in secretion from patients with ARDS compared to patients without. This phenotypic and functional PMN characterization can provide valuable diagnostic and therapeutic information for the intensive care treatment of ARDS patients.
Keywords: ARDS; ROS; flow cytometry; intensive care; neutrophil; surface epitope.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








References
-
- Read by QxMD Acute Respiratory Distress Syndrome: The Berlin Definition. [(accessed on 9 September 2023)]. Available online: https://read.qxmd.com/read/22797452/acute-respiratory-distress-syndrome-....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

