Plasmalogens Improve Lymphatic Clearance of Amyloid Beta from Mouse Brain and Cognitive Functions
- PMID: 39684263
- PMCID: PMC11640916
- DOI: 10.3390/ijms252312552
Plasmalogens Improve Lymphatic Clearance of Amyloid Beta from Mouse Brain and Cognitive Functions
Abstract
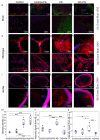
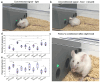
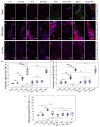
Amyloid beta (Aβ) is a neuronal metabolic product that plays an important role in maintaining brain homeostasis. Normally, intensive brain Aβ formation is accompanied by its effective lymphatic removal. However, the excessive accumulation of brain Aβ is observed with age and during the development of Alzheimer's disease (AD) leading to cognitive impairment and memory deficits. There is emerging evidence that plasmalogens (Pls), as one of the key brain lipids, may be beneficial for AD and cognitive aging. Here, we studied the effects of Pls on cognitive functions and the lymphatic clearance of Aβ from the brain of AD mice and mice of different ages. The results showed that Pls effectively reduce brain Aβ levels and facilitate learning in aged but not old mice. In AD mice, Pls improve the lymphatic clearance of Aβ that is accompanied by an increase in general motor activity and an improvement of the emotional status and learning ability. Thus, these findings suggest that Pls could be a promising candidate for the alternative or concomitant therapy of AD and age-related brain diseases to enhance the lymphatic clearance of Aβ from the brain and cognitive functions.
Keywords: Alzheimer’s disease; age; amyloid beta; cognitive functions; lymphatic clearance; plasmalogens.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

