Effect of Long-Term Cisplatin Exposure on the Proliferative Potential of Immortalized Renal Progenitor Cells
- PMID: 39684265
- PMCID: PMC11640886
- DOI: 10.3390/ijms252312553
Effect of Long-Term Cisplatin Exposure on the Proliferative Potential of Immortalized Renal Progenitor Cells
Abstract
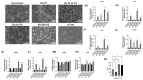
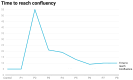
Cisplatin (CisPt) is a widely used chemotherapeutic agent. However, its nephrotoxic effects pose significant risks, particularly for the development of acute kidney injury (AKI) and potential progression to chronic kidney disease (CKD). The present study investigates the impact of non-lethal exposure of CisPt to immortalized human renal epithelial precursor TERT cells (HRTPT cells) that co-express PROM1 and CD24, markers characteristic of renal progenitor cells. Over eight serial passages, HRTPT cells were exposed to 1.5 µM CisPt, leading to an initial growth arrest, followed by a gradual recovery of proliferative capacity. Despite maintaining intracellular platinum (Pt) levels, the cells exhibited normal morphology by passage eight (P8), with elevated expression of renal stress and damage markers. However, the ability to form domes was not restored. RNA-seq analysis revealed 516 differentially expressed genes between CisPt-exposed and control cells, with significant correlations to cell cycle and adaptive processes, as determined by the Reactome, DAVID, and Panther analysis programs. The progenitor cells treated with CisPt displayed no identity, or close identity, with cells of the normal human nephron. Additionally, several upregulated genes in P8 cells were linked to cancer cell lines, suggesting a complex interaction between CisPt exposure and cellular repair mechanisms. In conclusion, our study demonstrates that renal progenitor cells can recover from CisPt exposure and regain proliferative potential in the continued presence of both extracellular CisPt and intracellular Pt.
Keywords: HRTPT; cell cycle; cisplatin; proximal tubule; renal progenitor cells.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Aldossary S.A. Review on Pharmacology of Cisplatin: Clinical Use, Toxicity and Mechanism of Resistance of Cisplatin. Biomed. Pharmacol. J. 2019;12:7–15. doi: 10.13005/bpj/1608. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

